Introduction
Clavicle fractures account for 2-10% of all fractures in adults, of which the majority occur in the middle third of the clavicle (Canadian Orthopaedic Trauma Society 2007a). Traditionally, these fractures have been treated nonoperatively (Canadian Orthopaedic Trauma Society 2007a). However, recent literature lacks a clear consensus regarding whether surgical or non-surgical management yields better outcomes in union rates and patient-perceived functional improvements (Canadian Orthopaedic Trauma Society 2007a; Heyworth et al. 2022; Axelrod et al. 2020). As a result, both options are routinely utilized in current practice. This lack of clarity in the approach to clavicle fractures has led to multiple studies in order to further delineate and compare the outcomes and cost-effectiveness of open reduction and internal fixation (ORIF) versus nonoperative management of these fractures (Canadian Orthopaedic Trauma Society 2007a; Heyworth et al. 2022; Axelrod et al. 2020; Nicholson et al. 2019; Liu et al. 2019).
Although rare, pneumothorax (PTX) is one of the most feared complications following surgical treatment of clavicle fractures. In one series of 1,350 patients who underwent ORIF of midshaft clavicle fractures, pneumothoraxes’ were identified in 16 patients (1.2%) (Leroux et al. 2014). Increased patient age and surgeries performed at an academic hospital were significantly associated with a greater odds ratio of PTX. Due to this feared complication, many surgeons routinely obtain chest x-rays (CXR) in the post-anesthesia care unit (PACU) following clavicle ORIF. Despite this increased utilization of CXR, the value of these x-rays has not been assessed. In the current healthcare climate where costs are greatly scrutinized, it is important to establish the utility and cost effectiveness of a diagnostic test.
The purpose of this study is to evaluate the cost-effectiveness of routine CXR after clavicle ORIF. Given the low incidence of PTX post-clavicle ORIF, we hypothesized that obtaining CXR would not be a cost-effective strategy.
Methods
This study was designed as a healthcare two-way dichotomous model (Figure 1). Decision trees were built where a CXR was either obtained or not. The clinical scenario most closely mimicking the model studied herein, represents an outpatient clavicle ORIF in the non-acute trauma setting, in a non-polytrauma patient. This scenario was chosen since most clavicle fractures are treated non-emergently and as an outpatient surgery in the United States (Yang, Werner, and Gwathmey 2015; Schairer et al. 2017; Pang et al. 2017). Moreover, given that CXR does not typically change clinical practice beyond PTX, no other complications were included in this study (Leroux et al. 2014; Shubert et al. 2019a).
Health States and Probabilities
The literature was queried to evaluate the incidence of PTX after clavicle ORIF. Provided the scarcity of data on PTX as an outcome of clavicle ORIF, our review was open to all types of articles ranging from case reports to systematic reviews and meta-analysis. Other data obtained from the literature included: cost estimates of CXR, cost of clavicle ORIF, length of stay for PTX treatment, rate of PTX treatment requiring more than observation, cost of PTX treatment and the health utility values of patients undergoing ORIF.
Literature reported incidence of PTX after clavicle ORIF demonstrates a rate of 1.2% (16 out of 1,350 patients). Of these 16 patients, 8 required chest tube insertion. In all instances the authors could not report whether the reported PTX occurred secondary to injury or due to surgical treatment which is significant given that an estimated 3% of patients with clavicle fractures also sustain a PTX regardless of surgical intervention (Leroux et al. 2014). Evaluation of the American Board of Orthopaedic Surgery part II data by Navarro et al. did not report any PTXs as a complication after ORIF for clavicle fractures undergoing surgical management in their evaluation of submitted cases (Navarro et al. 2016). A summary of the model parameters and their sources is outlined below (Table 1).
Given that most PTX are transitional states of disease of a worse health state for a defined period of time and not classified as chronic conditions, a discounted health utility state was calculated for those who sustained a PTX, which was 10% less of the utility of those 1 year out of a clavicle ORIF. Provided there is no reported health utility state value in the literature of patients who recovered from a PTX after clavicle ORIF, the 10% discounted health utility state is necessarily an estimated discounted value. Health utility states used were 0.91 one year after ORIF and 0.819 (0.91 – 10% = 0.819) one year after ORIF with PTX.
Costs and Cost Savings Estimation
The cost of ORIF, CXR and hospitalization were obtained from the literature (Table 2). For cost savings estimations the Pearldiver Database (Pearldiver Inc, Colorado Springs, Colorado) was queried for CPT-23515 (Clavicle ORIF) (Pang et al. 2017). Annual volume of procedures performed from 2010 to 2018 were obtained from the PearlDiver database using the CPT code above. The cost of developing a PTX was calculated based on average daily inpatient hospital stay costs from length of stay reported in the literature for this complication (Table 2).
To estimate potential cost savings of not obtaining CXRs, we elected to evaluate different rates of obtaining CXR after clavicle ORIF based on the annual number of clavicle ORIFs. Estimation of CXR rates were performed from 3% to 98% of patients undergoing clavicle ORIF and cost savings calculated as: Cost of CXR x n undergoing CXR = annual savings. Lastly, number needed to treat (NNT) was also evaluated regarding costs. The following formulas were used to calculate NNT.
Absolute Risk Reduction = (Control event rate) − (Experimental event rate)
NNT = 1/ARR.
Cost estimates were not adjusted for inflation during estimation of potential cost savings provided the short time frame and because costs would increase similarly between the different rates of ORIFs undergoing CXR used in our analysis. An estimation of cost savings was performed after an optimal strategy was decided based on the Montecarlo simulations (see below). A Montecarlo simulation is the preferred method of assessing the probability of different outcomes when random variables exist. Uncertainty is addressed by running the simulation numerous times to yield estimates that accurately approximate real-life circumstances.
Data analysis and Deterministic Sensitivity Analysis
The probabilistic sensitivity analysis with 2 arm decision tree and Montecarlo simulations were designed to evaluate the cost effectiveness of our clinical question. Given the lack of retrospective or prospective data on this subject, we elected to perform a deterministic sensitivity analysis that, represents multiple iterations (10,000) and computations of the probabilities (Table 1). The probability distribution of no PTX without CXR in PACU was estimated using a Beta distribution with a mean of 90% and standard deviation (SD) of 5% while the probability distribution of no PTX with CXR in PACU was based on a gamma distribution with mean of 95% and SD of 2%. These probabilities and their mean occurrence rates were chosen based on the sporadic occurrence of the outcomes of interest. Furthermore, the high SDs are reflective of the rarity of these events as well. The health utility values were used as an effectiveness marker. The commonly referenced threshold of $50,000 willingness to pay was used for this cost effectiveness analysis (Nwachukwu and Bozic 2015).
For estimation of the optimal clinical strategy to determine whether CXR in PACU was cost effective, the net monetary benefit (NMB) was chosen as the outcome of interest. Net monetary benefit (NMB) represents the most cost-effective strategy considering the costs and health utility outcomes.
The TreeAge pro Healthcare version 2021 R1.0 software (Williamstown, Massachusetts) was used for the modeling and simulation and all statistical analysis. Microsoft Excel (V16.4, Redmond, Washington) was used to compute cost savings. Data obtained from the PearlDiver Server was extracted from the Mariner dataset which is held within a HIPAA compliant database that de-identifies patients; therefore, an IRB review was not indicated.
Results
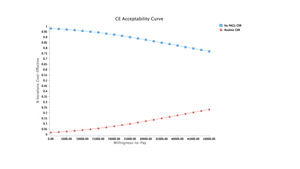
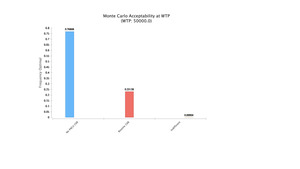
Montecarlo simulations proved ORIF of the clavicle without CXR in the PACU to be the most cost-effective option with a NMB of $32,022.50 (Figure 1). Payoffs with each of the reported strategies after model rollback are further demonstrated (Figure 1). Moreover, at the chosen willingness to pay (WTP) threshold of $50,000, the no CXR strategy was the dominating option. Comparisons of the strategies can be seen with the WTP curve (Figure 2).
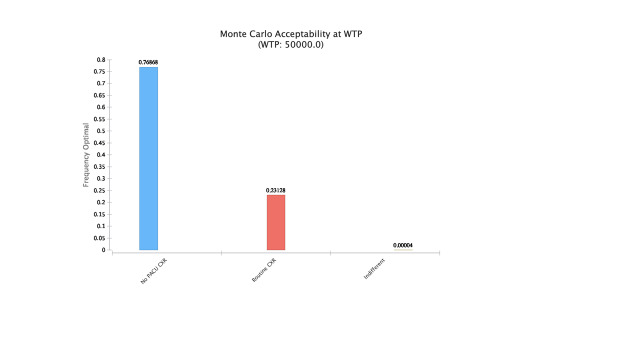
The results of the Montecarlo simulations with cost effectiveness at different thresholds of WTP based on chosen strategy are shown below (Figure 3). As WTP increases, obtaining CXR becomes cost effective following ORIF. The no CXR strategy was found to be optimal 76% of the time at WTP of $50,000 followed by routine CXR 23% of the time and less than 1% being indifferent (Figure 4).
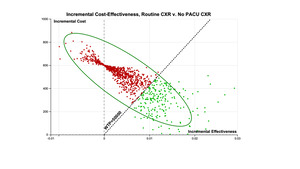
The incremental cost effectiveness comparison of the two strategies is depicted below (Figure 5). The repetitive simulation of the events demonstrates the low incremental effectiveness that is obtained from routine CXR after ORIF (red region), and the green region represents the more cost-effective strategy of no PACU CXR.
Estimation of Possible Cost Savings
From 2010 to 2018 there were a total of 9,060 clavicle ORIFs identified within the PearlDiver Database. The mean annual number of ORIFs was 1,006 (SD 143.4). These procedures represented $38,369,304 in reimbursements.
The national annual cost of routine CXR after clavicle ORIF was estimated to range from $7,100 to $349,860 (3% vs 98% undergoing CXR, respectively), which over 10 years represents anywhere from $81,540 to $2,663,640. If no CXR had been obtained for all the ORIFs performed from 2010 to 2018, $2,718,000 would have been saved. As described by Leroux et al. 1.2% were found to have a PTX based on CXR, this would incur a cost of $32,616 for 108.7 ORIFs (Leroux et al. 2014) . If CXR was able to identify all PTX after clavicle bringing the missed incidence to 0, then 83 CXR would be the NNT, representing a cost of $24,900 (83 x $300.0).
Discussion
Clavicle fractures are a common occurrence in the United States. While the literature remains divided on whether surgical fixation or non-operative management is the preferred treatment strategy, high-level studies have shown the possible benefit of higher union rates with clavicle ORIF (Canadian Orthopaedic Trauma Society 2007a). As such, the rate of clavicle ORIF remains around a thousand cases performed annually. One of the more feared complications of clavicle ORIF is postoperative PTX. To avoid a missed PTX, many surgeons cautiously order CXR in the PACU after clavicle ORIF. The value and associated cost of this routine CXR in PACU following ORIF of clavicles has not been reviewed.
The model without CXR proved to be the most cost-effective strategy. Net monetary benefit (NMB) is a method of expressing cost effectiveness that considers both the willingness to pay threshold and health benefits (QALYs) for a given intervention. By converting QALYs into monetary units, the cost of each treatment strategy can then be deducted. With a NMB of $32,022.50 and at a WTP of $50,000, the no CXR strategy was the preferred option (Figure 1). 76% of the time, this strategy was found to be optimal followed by routine CXR 23% of the time and less than 1% being indifferent (Figure 4). The national annual costs of routine CXR after clavicle ORIFs were estimated to range from $7,100 to $349,860, which over 10 years could have represented anywhere from $81,540 to $2,663,640 based on annual percent imaged.
Despite the presented cost-effective findings after forgoing post-operative CXR, surgeons may be reluctant to adopt this practice and should continue to use their clinical judgement in regard to the need for postoperative CXR. Risk factors for PTX such as infraclavicular blocks, or worsening emphysema, are well documented in the literature and can help guide surgeons in determining which patients would most benefit from a CXR in the PACU following clavicular ORIF (Gauss et al. 2014). Other indications such as pleuritic chest pain or changes in vitals in the operating room or PACU, could also be used as an indication for postoperative CXR. Acknowledging these very real and legitimate reasons to obtain a postoperative CXR for clavicle ORIF, physicians should continue to use clinical judgement in the PACU regarding the need for a CXR.
In addition to the risk factors noted to increase incidence of PTX following clavicle ORIF mentioned above, several other considerations are worth discussing. First, is the predicted sensitivity of CXR in identifying PTX. Recent studies have found that CXR had a lower pooled sensitivity of detecting PTX, while chest ultrasound (CUS) demonstrated a significantly improved pooled sensitivity in comparison (Ebrahimi et al. 2014; Alrajab et al. 2013). This presents an alternative more cost-effective strategy than obtaining post-operative radiographs to help guide surgeons in patients with signs concerning for PTX (Alrajab et al. 2013). As mentioned previously, monitoring of intraoperative vital signs can assist in raising suspicion for PTX. Changes in airway pressures or hemodynamic stability could suggest PTX, in which intraoperative fluoroscopy has successfully been used to diagnose PTX (Heyba, Rashad, and Al-Fadhli 2020). While these alternative methods have been shown to successfully detect PTX, their true cost-effectiveness and practical utility in clavicle post-ORIF is harder to infer.
Our study is not without limitations. The data used for this analysis is based on a retrospective review of the literature and point estimates. It is likely that the individual studies referenced held different protocols in determining the incidence of post-operative complications of pneumothorax. This includes limitations in identifying whether pneumothorax was secondary to the initial presenting injury or due to ORIF, and identification of delayed presentations of PTX beyond the post-operative hospital monitoring period. There is also a possibility that PTX is underreported due to potential inaccuracies in diagnosis. Despite this, we anticipate minimal variation in the reported incidence and expect no significant impact on our reported outcomes of cost effectiveness strategies.
Further, limitations exist in regard the need for estimations of procedural cost and health utility discounts. Regional and institutional costs may vary for imaging studies and operative procedures outside of our reported ranges. A discounted health utility state was assigned for those who sustained PTX at 10% less of the utility of those one year out of clavicle ORIF. Given the paucity of literature referencing utility states of individuals recovering from PTX after clavicle ORIF, this estimation may over or undervalue the true discounted utility rate. Moreover, the cost estimates of not obtaining CXR and developing a PTX leading to severe long-term morbidity or mortality have not been included in our model given the rarity of these events. As such, our data warrants prospective studies to validate our findings. Nonetheless, our study offers annual and 10-year approximations of cost savings across several decision strategies, with clear evidence that the no CXR option yields the most financial sense. By referencing numerous smaller, single-institution studies in our literature review for cost and outcomes data, our results are substantiated and allow hospitals and surgeons to best allocate resources and eliminate expenditures to patients.
The distribution of the health states of patients who receive postoperative CXR after clavicle ORIF is great, spanning from young active adults to elderly patients with varying degrees of comorbidities, associated injuries and inciting traumatic events. Future studies should investigate patient associated injuries and comorbidities that would place a patient at higher risk for PTX after clavicle ORIF with the goal of elucidating whether postoperative CXR is appropriate. Nonetheless, our results demonstrate that for the majority of patients undergoing clavicle ORIF, routine PACU CXR is not warranted.
Conclusion
Routine CXR following clavicle ORIF may be an example of defensive medicine, where fear of potential pneumothorax and a poor patient outcome causes reflexive ordering of post-operative radiographs. We have found that this practice is neither cost-effective, nor in most cases, clinically necessary. Elimination of PACU CXR represents an opportunity for cost savings at the institutional level as well as limiting excessive expense on behalf of the patient.
We recommend that surgeons use their best clinical judgment in deciding whether to order CXR in high-risk patients or those symptomatic post-procedure, as certain instances do warrant investigation. For the vast majority of asymptomatic patients, we believe following the no CXR strategy is the more appropriate clinical and fiscal option.