Introduction
Shoulder arthroplasty surgeries have become more prevalent in the United States over the past three decades. There was a 720% increase in the prevalence of patients who underwent total shoulder arthroplasty from 1995 to 2017 (Farley et al. 2021). Contributing to this is an increased use of reverse total shoulder arthroplasty (rTSA) (Wagner et al. 2020). This increased prevalence of shoulder arthroplasty patients has led to a subsequent increase in the revision shoulder arthroplasty (Singh, Sperling, and Cofield 2011).
Failure of total shoulder arthroplasty can result from infection, instability, loosening, and soft tissue deficiencies (Chalmers et al. 2019). Periprosthetic joint infection (PJI) is a particularly devastating cause of shoulder arthroplasty failure and often difficult to salvage. Treatment may include debridement, single stage revision, two-stage revision, arthrodesis, chronic antibiotic suppression, placement of permanent spacer, and amputation (Bonnevialle et al. 2017). When revision reconstruction is to be considered, conventional options include hemi-arthroplasty, osteoarticular allograft, and allograft prosthetic composite (APC), each of which has its own limitations and risks.
Custom constrained total shoulder (CCTS) endoprosthesis is a reconstructive alternative for difficult to salvage cases particularly after failed shoulder arthroplasty with glenoid and humeral bone loss. Advantages of custom implants include the ability to match remaining anatomy, accommodate unique bone deficiency, achieve stability, restore the joint line, optimize prosthesis orientation, and achieve screw placement with proper length and optimal positioning (Porcellini et al. 2021). Other cases of custom shoulder arthroplasty revisions have been reported (Porcellini et al. 2021; Chammaa, Uri, and Lambert 2017; Bodendorfer et al. 2021; Ortmaier, Wierer, and Gruber 2022; Debeer et al. 2019; Stoffelen, Eraly, and Debeer 2015), but none have used a bi-flanged scapular (BFS) component affixed to the remaining scapular body. We present two cases of CCTS with BFS endoprosthesis to salvage failed shoulder arthroplasty secondary to PJI.
Case 1
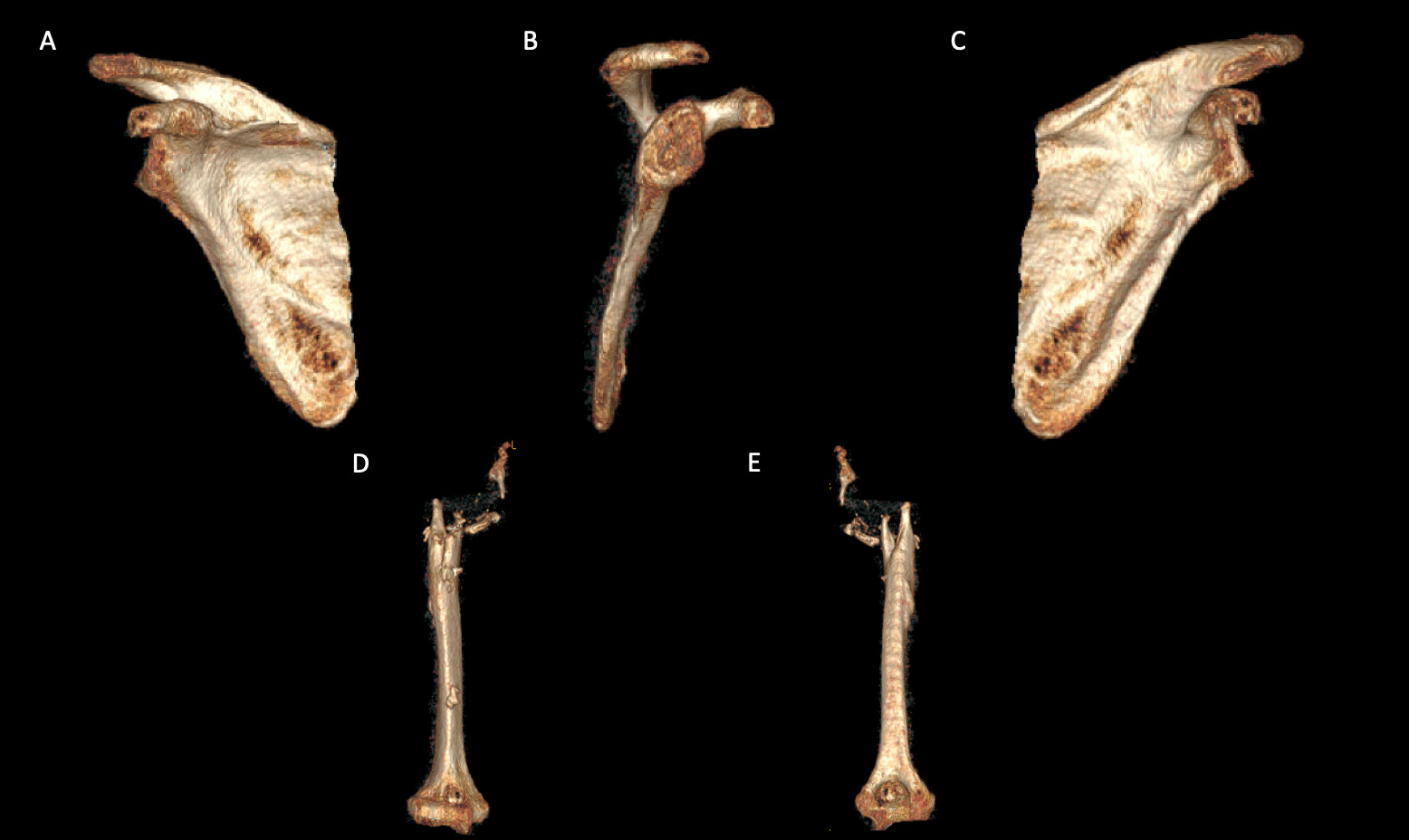
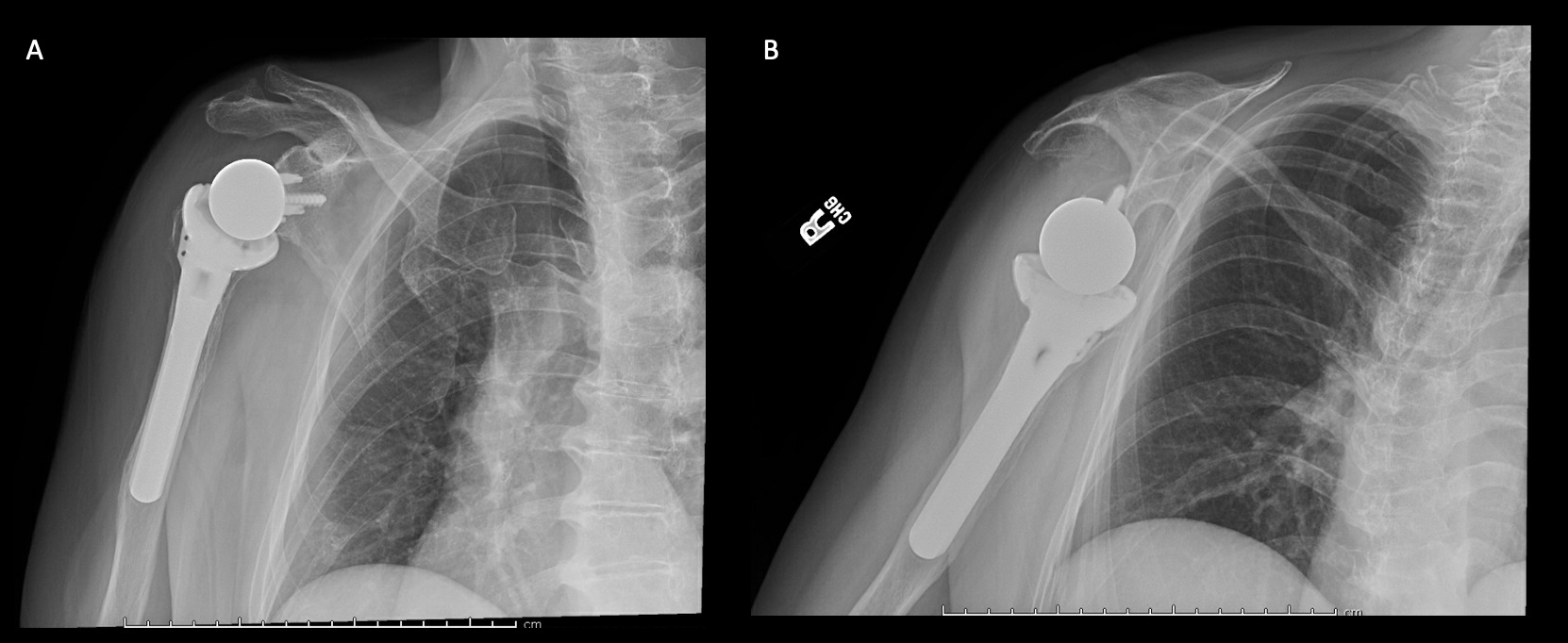
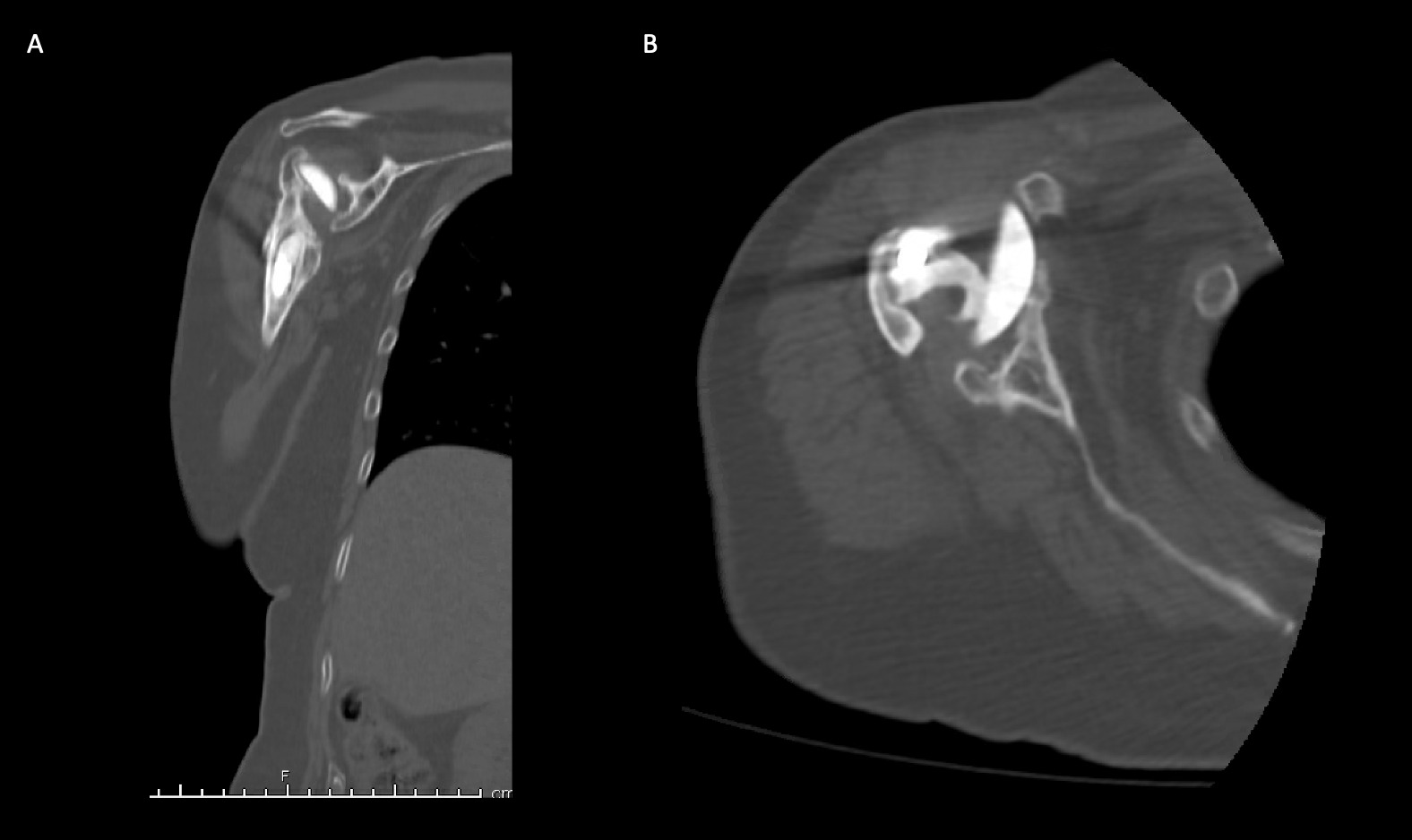
A 69-year-old woman presented after resection arthroplasty of her right shoulder hemiarthroplasty and completion of antibiotic treatment for culture negative PJI for evaluation of reconstructive options. She had a history of a displaced two-part right proximal humeral fracture which was repaired with an intramedullary nail. Post operatively, she had persistent pain and CT scan of the shoulder showed two screws in the humeral head that extended through the cortex and into the glenohumeral joint. The screws were removed and replaced with smaller screws. Subsequently, she had non-union of the fracture in the setting of deltoid weakness and was revised to a right hemiarthroplasty (Figures 1-3). Post operatively, she had persistent pain refractory to physical therapy and diminished function that was impacting her quality of life. She sought subsequent care, and there was concern for arthroplasty failure secondary to a septic versus aseptic condition and the decision to proceed with resection arthroplasty was made. During resection arthroplasty, a significant amount of fluid was expressed from the joint space. The combination of the clinical picture of progressive and persistent pain and the gross expression of fluid were concerning for PJI and the decision to treat was made. It was felt that the extent of humeral bone loss did not allow for a stable cement spacer, so none was placed. Intraoperative joint fluid cultures, anaerobic, and fungal cultures were all negative for growth. Cultures were held for 21 days to rule out C. Acnes infection. Considering the plan for future reimplantation, infectious disease consultation recommended a 6-week course of vancomycin but after 2 days the patient had a transfusion reaction and was switched to daptomycin. She was left with markedly limited active motion associated with severe pain. After completion of the antibiotic course, the patient elected to proceed with revision surgery. This patient had a flattened and distorted glenoid and atrophied rotator cuff musculature demonstrated prior to resection arthroplasty on CT (Figure 4). This patient’s prior hemiarthroplasty had been a tantalum coated, cemented humeral stem with associated marked thinning of the humeral cortex even prior to resection arthroplasty, and this resulted in a large amount of proximal humeral bone loss during the resection (Figure 5). The patient was severely debilitated by the right shoulder and wanted to proceed with reconstruction. Because of the combination of glenoid and humeral bone loss and the lack of rotator cuff attachments, a custom constrained prosthesis design process was initiated, and the implant was available 16 months after the resection arthroplasty. Preoperative 3 phase SPECT (Single Photon Emission Computed Tomography) three phase bone scan, upper extremity CT, and blood work (WBC 5.4, ESR 9, CRP 0.8) showed no suggestion of active infection.
Case 2
A 62-year-old woman presented for evaluation of her reconstructive options after resection arthroplasty of a right rTSA and completion of antibiotic treatment for culture negative PJI. Her rTSA had been an Encore prosthesis placed due to rotator cuff arthropathy (Figures 6). Post-operatively she had persistent pain, stiffness, and tenderness to palpation that was treated with physical therapy, a steroid injection, and manipulation under anesthesia. An infectious work up due to the chronic and persistent nature of the pain showed WBC 7.4, ESR 53, CRP 1.2. Patient denied further work up with a tagged white cell scan and preferred continued clinical monitoring to observe pattern of symptoms. Eleven months later, she endorsed persistent pain. Repeat infectious work up showed WBC 4.5, ESR 13, and CRP 1.1. Despite the normal inflammatory markers, a 10-day trial of Augmentin was initiated and failed to change her symptoms. The decision to proceed with 1 vs 2 stage reconstruction was made. During resection arthroplasty, a substantial amount of fluid was expressed from the joint capsule concerning for PJI. A cement spacer was placed, and the patient was treated with 6 weeks of vancomycin and cefepime (Figure 7). There was proximal humeral bone loss as the result of resection arthroplasty due to the humeral stem. The glenoid component was loose and associated with glenoid bone loss, further complicated by the need for removal of a broken central screw (Figure 8).
Intra-operative frozen sections, tissue cultures, and gram stain showed no signs of infection. Following treatment, inflammatory markers remained normal (WBC 5.5, ESR 22, CRP 0.9), and aspiration of the shoulder showed no organisms on gram stain and no growth on aerobic, anaerobic, fungal, and acid-fast bacilli cultures. Cultures were held for 21 days to rule out C. Acnes infection. She was left with profoundly limited active motion and severe pain and the patient elected to proceed with revision surgery. Because of the combination of severe glenoid and humeral bone loss and the lack of rotator cuff attachments necessitating a design with additional constraint, the decision to proceed with custom prosthesis design was made 9 weeks after resection arthroplasty.
Prosthesis Description and Manufacturing
The prosthesis in both cases was a constrained articulation between the scapular and a humeral component. The scapular component was a custom bi-flanged glenoid component with anterior and posterior flanges that wrapped around the scapular body to which a Trident locking mechanism was attached. The humeral component was composed of a modular replacement system humeral stem with a conformation like a proximal femoral component with a male taper to which a femoral head was attached.
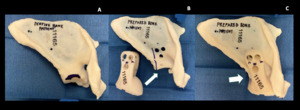
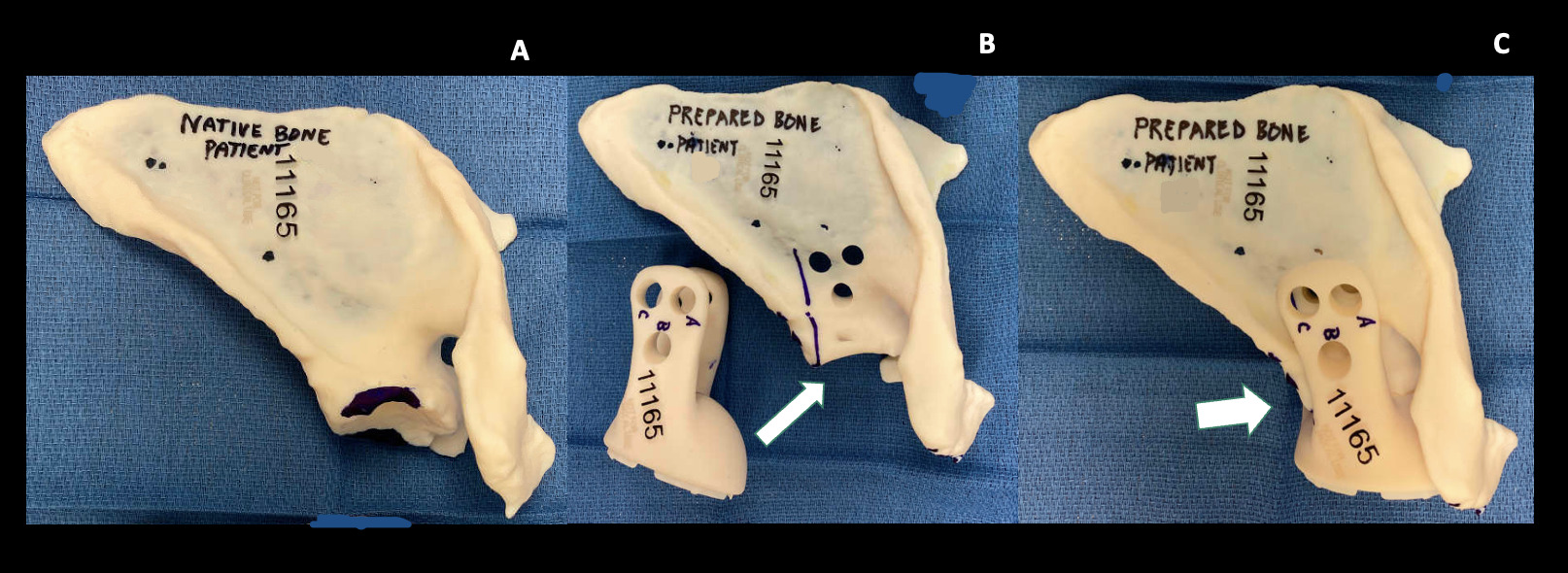
The prosthesis custom design was based on pre-operative protocol CT scan through joint meetings of the primary surgeon (TAD) and the design team (Stryker, Inc., Mahwah, New Jersey, USA). Once design plans were finalized, outside independent review of the surgical and custom endoprosthetic indication was received, and Institutional Review Board approval was obtained under the Compassionate Use protocol, the endoprosthetic implant was produced by a proprietary 3-D printing process (Stryker). In addition to the implant, a 3D constructed replica of the patient’s native and post-preparation scapula(individually), a replica of the bi-flanged custom glenoid component, and replicas of the custom threaded drill guides were manufactured with a plastic resin that allowed them to be sterilized and used for anatomic reference during the procedure (Figure 9). The regions of the native scapula that required contouring with a burr were marked on the model.
Operative Indications
Both patients had a history of failed shoulder arthroplasty resulting in substantial humeral and glenoid bone loss along with soft tissue loss. CCTS with BFS endoprosthesis was indicated due to the anatomical deficiencies. The constraint was needed to address the lack of adequate soft tissues to produce a reliably stable glenohumeral articulation. The substantial glenoid replacement was felt necessary to replace deficient scapular bone and the flanges to provide stable fixation to withstand the stresses of a constrained glenohumeral articulation.
Surgical Technique
In each case, the patient was placed in a semi-beach chair position. The initial anterior approach was through the deltopectoral interval via the old scar. Posteriorly, a transverse incision was made in the infrascapular spine region to expose the posterior scapula for placement of trans-scapular bolts through the glenoid component from posterior to anterior. Irrigation and extensive debridement of both the intraarticular and intramedullary canal of the proximal humerus was performed, given the history of prior PJI. Debridement was performed utilizing rongeurs, curettes, elevators, and a scalpel. To expose the anterior scapula, a standard deltopectoral was used for proximal humeral and scapular exposure. Subperiosteal dissection was accomplished beneath the remaining capsule both anteriorly and posteriorly at the level of the glenoid, thereby avoiding injury to the suprascapular nerve cephalad to this region and the quadrilateral space inferiorly. Following the inferior glenoid, subperiosteal dissection was also accomplished along the lateral scapular border. The latter was accomplished to facilitate rotating of the flanges onto the scapular body from inferiorly to their final resting horizontally oriented position. Once the flange was positioned on both sides of the scapular body, the posterior flange was exposed through a separate posterior incision beginning on the posterior aspect of the acromion and extending medially. The deltoid was detached from the mid portion of the scapular spine beginning lateral to the scapular notch and extending to the posterior lateral aspect of the acromion. A cuff of deltoid and periosteum was preserved for subsequent repair.
Because of associated severe proximal humeral bone deficiency in case 1, a femoral strut allograft and 3 cables were used to cover the defect to contain the intramedullary humeral canal to accept the cemented intramedullary stem. In case 2, humeral prosthesis trialing required resecting 2 cm of bone distal to the original cut of the proximal humerus to accommodate the proximal humeral replacement endoprosthesis standard body.
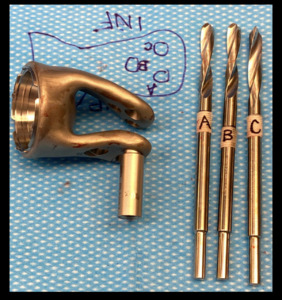
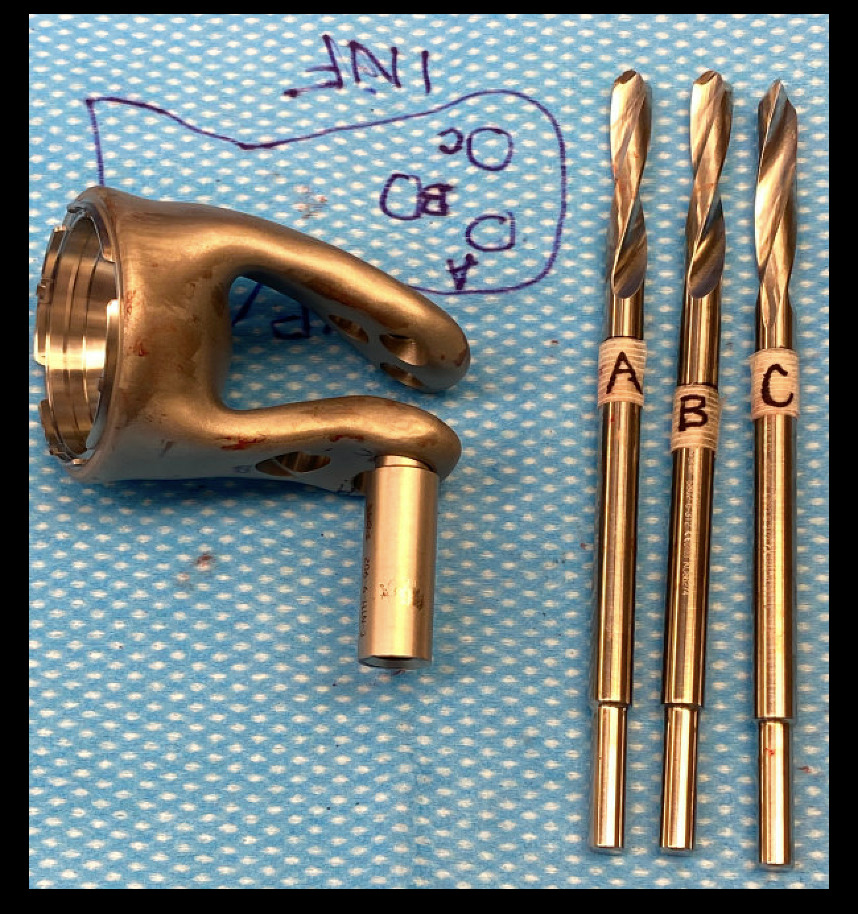
To optimize apposition of the custom flanges onto the anterior and posterior aspects of the remaining scapular body, a small amount of the remaining glenoid was contoured with a high-speed burr using a CT-based sterile model of the desired shape as a reference (Figure 9). Once optimal fit and apposition were achieved, three posterior to anterior bolts were used to affix the anterior and posterior BFS arms to the scapula. Drill bits were marked intraoperatively, using the provided model of the endoprosthesis and drill guides, with a steristrip as a visual cue for the planned depth for drilling of each of the three holes flange holes to avoid damaging the threads in the holes on the deep anterior flange of the prosthesis since the far side could not be directly visualized (Figure 10). Each custom drill guide was screwed into the corresponding posterior hole of the flange (Figure 11). After drilling, each bolt was passed through the posterior flange of the prosthesis, the scapular bone, and then to engage the threads in the anterior flange. Bone cement was introduced from the superior aspect of the scapula to fill gaps between the flanges and the underlying scapular body where necessary. To repair the deltoid sleeve, the sharp point of a suture needle was used to make holes through the scapular spine which the deltoid was tied over the top through the holes using #5 Ticron suture.
The humeral component was composed of a Stryker modular replacement system (MRS) humeral stem with a conformation like a proximal femoral component with a male taper (Figure 12). An actual femoral head was impacted onto the proximal humeral component. After cementing of the humeral component, a hex dome hole plug was inserted to cover the central glenoid hole. A constrained Trident acetabular insert was then impacted into the custom glenoid component, and the femoral head was reduced into the acetabular liner.
Pearls and Pitfalls
A floppy lateral position is key to attain adequate simultaneous anterior and posterior exposure of the scapula. Subperiosteal dissection about the glenoid is important to avoid injury to vital structures and difficult to control bleeding. Utilizing a Cobb or other curved ended periosteal elevator is helpful to allow elevation anteriorly and posteriorly onto the glenoid body and to facilitate palpation of the posterior flange through the posterior incision. Enough inferior exposure must be obtained to allow rotation of the two flanges from inferiorly to superiorly to engage the scapular body. Care must be taken throughout the procedure to remain on the scapular body side of the coracoid process to minimize risk to the brachial plexus, artery and vein.
Post-Operative Course
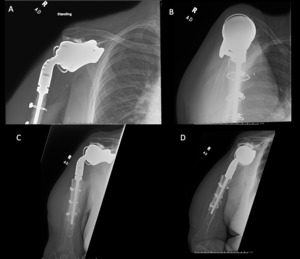
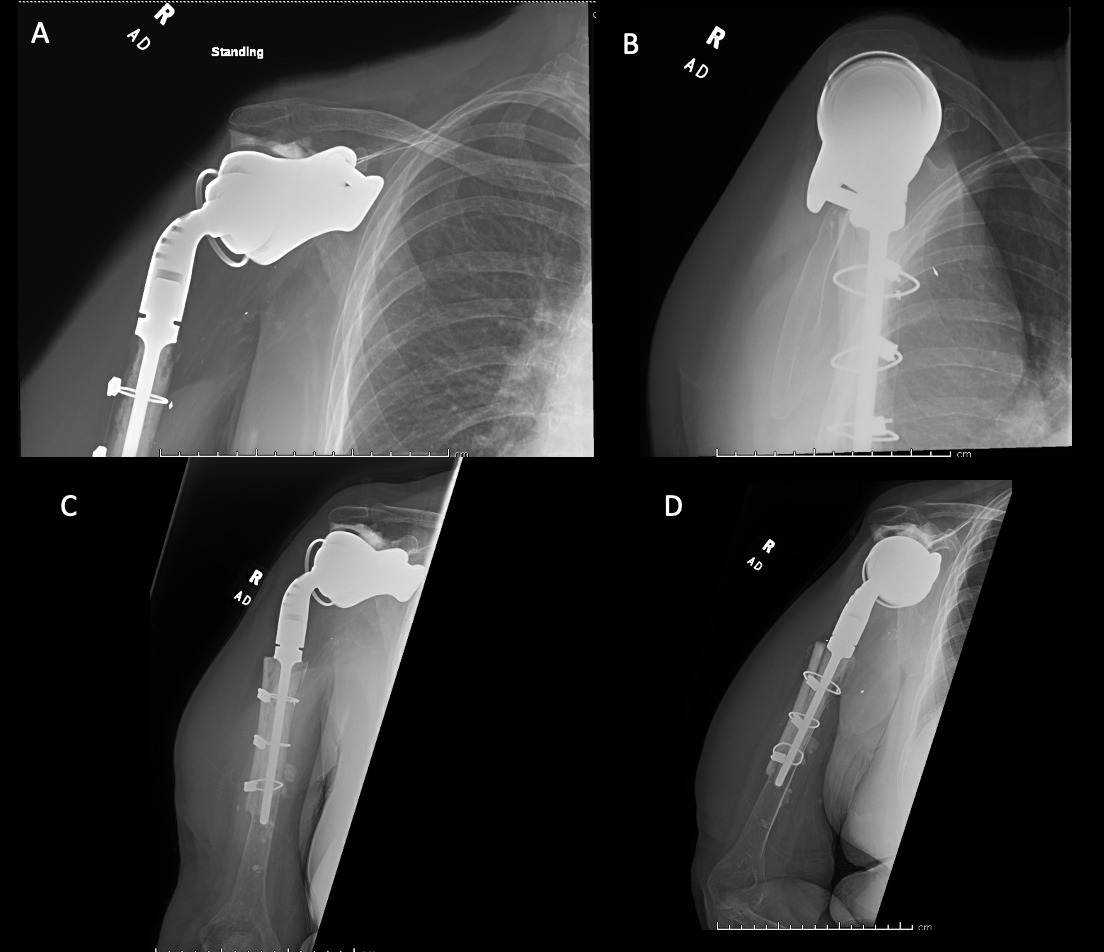
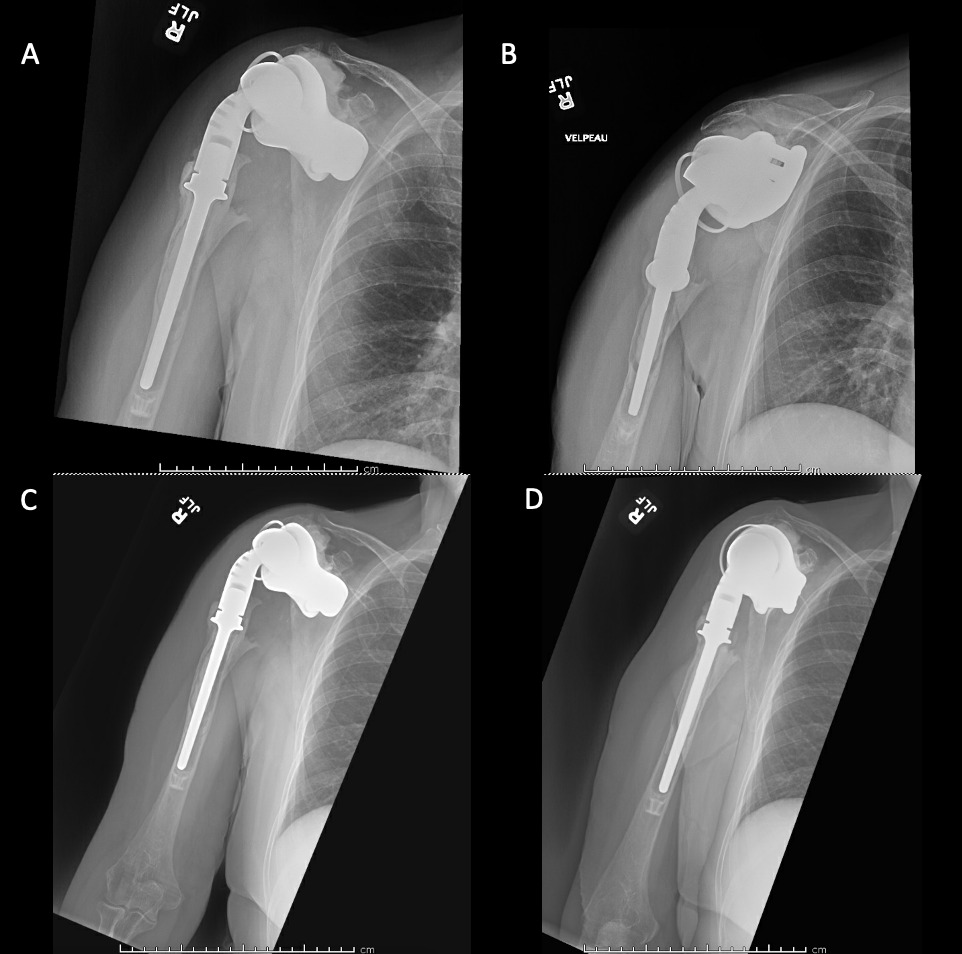
Case 1. At the latest follow-up one-year post-op, patient 1, who had the more extensive humeral exposure, was extremely pleased with the results of the surgery despite occasional pain. She had resolution of an early post-op radial nerve palsy. She could button up her blouse and get her hand to her mouth. Her shoulder and scapular x-rays showed some new radiolucency between the superior aspect of the glenoid prosthetic component and the associated bone cement in the subacromial space (Figure 13). At 2 years post-op she continues to have significant pain relief and has maintained her active range of motion (AROM). Elbow AROM was from 10 to 145 degrees. Shoulder AROM was abduction to 15 degrees and internal rotation allowing the hand to reach the level of L5/S1 vertebrae.
Case 2. At the latest follow-up one-year post-op, the patient expressed that the pre-operative pain had resolved after the operation. Despite this, she stated that if given the option again she would not undergo the surgery because she did not attain the function she expected and felt that her function may have decreased compared to pre-op. Overall stability and alignment were good. On physical exam, she was able to reach within 1 inch from her mouth and had 20 degrees abduction and 5 degrees forward flexion actively. Active elbow flexion was 0 to 120 degrees (Figures 14). At 2 years post-op she had some regression in her pain but accompanied by modestly decreased AROM. Shoulder AROM was 15 degrees abduction, and 5 degrees forward flexion. Elbow AROM was from 0 to 105 degrees of elbow flexion. CT scan showed increased lucency surrounding the humeral component concerning for osteolysis and prosthesis loosening (Figure 14). Blood work at the 2-year mark to investigate the radiolucencies and decreased AROM left us with low suspicion for infection (WBC 5.4, ESR 33, CRP <3.0).
Discussion
Given the recent rise in the prevalence of shoulder arthroplasty patients, the need for revision shoulder arthroplasty has increased (Farley et al. 2021; Singh, Sperling, and Cofield 2011). Custom endoprostheses have been reported infrequently for this indication, and none of the reported designs had a custom constrained total shoulder (CCTS) with a bi-flanged scapula (BFS) extending both anterior and posterior to the scapular body. In this study, two cases of revision shoulder arthroplasty with a novel CCTS with BFS endoprosthesis are reported.
Other custom shoulder endoprosthetic designs have been reported to address substantial glenoid bone loss that leaves inadequate glenoid on which to base the scapular reconstruction similar to the reported two cases (Table 1). In the design that is most similar to the current report, Chaama et al. described a custom total hip inspired, large acetabular like glenoid shell affixed to the scapula around a deficient glenoid, but, instead of flanges as in this reported design, relied upon screw fixation directly through the shell into the remaining scapula (Table 2) (Chammaa, Uri, and Lambert 2017). Their design showed promising clinical improvement in patients with severe glenoid deficiencies in which decreased glenoid bone stock limits options for direct attachment of an endoprosthesis to the glenoid itself and allows for attachment directly to the scapula around the deficient glenoid. Revision was necessary in 6 of 37 patients (16%) at mean 60 months follow-up (Chammaa, Uri, and Lambert 2017).
Custom glenoid baseplates show promise for those with sufficient glenoid bone stock for direct fixation of a custom baseplate to the glenoid itself but with morphology or bone stock non conducive to reshaping for standard glenoid endoprostheses. This is as opposed to the substantial glenoid bone loss seen in our patients for which custom glenoid based arthroplasty is insufficient which required a custom glenoid component to wrap around the deficient glenoid. In this regard, Bodendorfer et al described a computer-aided design / computer aided manufacturing (CAD/CAM) custom glenoid base plate composed of plasma spray titanium that matches and contours to the patient’s specific glenoid defect and attaches to the glenoid itself for reverse shoulder arthroplasty (Table 2) (Bodendorfer et al. 2021). All measured clinical outcomes showed improvement in the median scores and of the 12 shoulders done all were radiographically stable without loosening and had no complications (Table 1). Porcellini et al. described a CAD/CAM reverse custom glenoid baseplate matching and contouring to the patient’s glenoid defects and attaching directly to the glenoid itself (Table 2). Improvements in clinical outcome scores and pain were reported in a cohort of 6 patients (Table 1) (Porcellini et al. 2021).
Several papers report glenoid endoprostheses in which a flat metal glenoid baseplate is attached to a custom porous metal structure which fills an irregular glenoid cavity. Ortameir et al. reported improvement in all clinical scores in their 10 patients (Table 1) (Ortmaier, Wierer, and Gruber 2022). Debeer et al. reported improved function in 8 of 10 patients (Debeer et al. 2019). Stoffelen et al. reported a case with increased range of motion and clinical score utilizing a similar design (Table 1) (Stoffelen, Eraly, and Debeer 2015).
The described CCTS with BFS endoprosthesis is effective in achieving fixation when the glenoid bone loss prevents fixation through the glenoid alone. In our two cases, pain relief was achieved in both but increased function in only one compared to resection arthroplasty with a spacer. This custom endoprosthesis represents an alternative reconstructive option for cases with substantial glenoid bone loss necessitating a constrained design. Currently, the construct shows limitation in its ability to provide post-operative improvement in function and range of motion. The constrained design may have contributed to hardware loosening seen in the second case, The small sample of patients who have received the construct limits the ability to draw conclusions regarding its role in reconstruction in the future, although the results are promising for achieving pain relief in salvage procedures. Long term follow up is needed to determine the viability of this construct.


_and_scapular_y_(b)_radiographs_show_hemi-arthroplasty_prior_t.png)
_and_axial_(b)_ct_images_prior_to_resection_arthroplasty_.png)




_and_anterior_with_internal_rotation_(b)_radiogr.png)




__axial_(b).png)


_and_scapular_y_(b)_radiographs_show_hemi-arthroplasty_prior_t.png)
_and_axial_(b)_ct_images_prior_to_resection_arthroplasty_.png)


_and_anterior_with_internal_rotation_(b)_radiogr.png)


__axial_(b).png)
