Roman Gladiators to American Engineers: Heat Sterilization Over 2000 Years
Medical instrument sterilization roots date back to the Roman gladiators (Figure 1). Galen was a Greek philosopher and physician (129-216 AD) who was known for successfully treating wounded gladiators. Unlike other surgeons, he would boil his surgical instruments before use. However, the reason for heat’s antiseptic effects was unknown at that time (West 2014; Steris 2018a).
After a long pause in the dark ages, a drapery practice in the 1600’s helped restart germ theory (Steris 2018a, 2018b). To evaluate fabric quality, merchants used magnifying lenses to count the threads in woven linen cloth. In 1683, a Dutch draper Antonie Van Leeuwenhoek developed the microscope for more powerful evaluation (Britannica 2022). He observed various microorganisms and is credited as the “father of microscopy” (Steris 2018b).
In 1862, Louis Pasteur a French chemist proposed the germ theory of disease (Ullmann 2022). He postulated microorganisms can lead to disease and that heat can be used to kill bacteria. The pasteurization process reduced food spoilage. Pasteur also noted that moist heat was more effective than dry heat for killing bacteria (Steris 2018b).
In 1876, Charles Chamberland, a pupil and colleague of Louis Pasteur’s, developed the first pressure steam sterilizer or autoclave. Pressurized steam has a higher boiling point and can more effectively carry heat. The word sterilization is introduced in 1874, over 2000 years after Galen boiled his medical instruments for gladiators (Steris 2018b).
In the 1930’s, German and American engineers help create the modern autoclave used today with temperature, vacuum and pressure control systems (Steris 2018b). The autoclave’s steam sterilization is the most dependable and commonly used method for medical instrument sterilization. It is affordable, nontoxic, and effective at deactivating bacteria, viruses, fungi and spores (Joslyn 2001; Association of PeriOperative Registered Nurses 2019; Centers for Disease Control and Prevention 2016).
How the Modern Autoclave Works
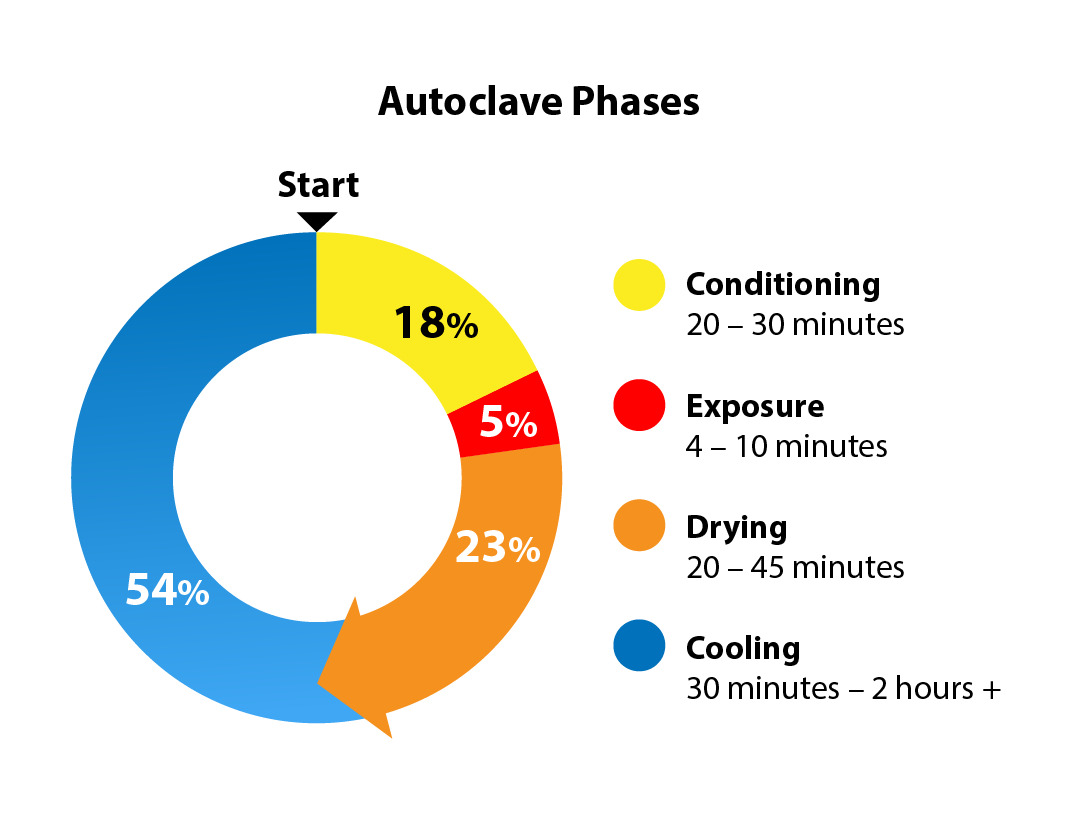
The modern autoclave exposes an item directly to steam for heat sterilization. Saturated steam (water content of ≤3%) is ideal for sterilization (American National Standards Institute Inc. and Association for the Advancement of Medical Instrumentation 2017; Centers for Disease Control and Prevention 2016). Pressurized steam allows for higher temperatures and therefore greater microbicidal efficacy. The modern autoclave regulates temperature, steam, pressure, and time and works in 4 phases: conditioning, exposure, drying and cooling (Centers for Disease Control and Prevention 2016) (Figure 2).
Conditioning builds the microbicidal environment (Figure 3). Air is removed from the sealed autoclave. Air is a poor sterilization medium and must be replaced with saturated steam (Association of PeriOperative Registered Nurses 2019; American National Standards Institute Inc. and Association for the Advancement of Medical Instrumentation 2017). Air can be removed passively (gravity displacement) or dynamically (vacuum suction or steam flushes).
A boiler introduces pressurized steam into the autoclave until the desired parameters have been achieved. The conditioning process typically takes 20-30 minutes.
Next, in the exposure stage, the appropriate temperature and pressure are held with direct steam exposure for the required time for microbial deactivation (Figure 4). The minimal kill time or adequate exposure time for a prevacuum sterilizer is 4 minutes at 270-275°F (132-135°C) (Centers for Disease Control and Prevention 2016; American National Standards Institute Inc. and Association for the Advancement of Medical Instrumentation 2017). It is the shortest stage.
After appropriate exposure, steam is removed from the autoclave chamber in the drying phase (Figure 5). Vacuum suction removes the steam and creates a subatmospheric negative pressure environment in the autoclave. A porous barrier is necessary to separate the contents of the sterilized trays from the external environment. The porous barrier allows the ingress and egress of steam from the autoclave into the sterilized trays while creating a sterile microorganism barrier. This is often a porous blue wrap used around a container, or a paper-thin filter used on the lid or body of a rigid container (Figure 6).
The last phase is the cooling phase (Figure 7). The autoclave is opened and contents are gradually and naturally allowed to cool to room temperature before touching or handling. The American National Standards Institute Inc (ANSI) and the Association for the Advancement of Medical Instrumentation (AAMI) carefully and explicitly state in a one-line paragraph, “Items should not be touched during the cooling process” because of the importance of sterile compromise with early handling (Association of PeriOperative Registered Nurses 2019; American National Standards Institute Inc. and Association for the Advancement of Medical Instrumentation 2017).
During the cooling phase, the moisture in the steam continues to escape the sterilized contents as water vapor through the porous barrier. Touching the hot porous barrier during the cooling phase may cause focal condensation and wick in bacteria from the hands into the sterile package compromising sterility. An infrared gun is commonly used to check the temperature before touching (Figure 8). Cooling can take 30 minutes - 2 hours or more depending on load and conditions. It is the longest stage of the steam sterilization process and cannot be forced.
The Problem with Modern Steam Sterilization: The Porous Barrier and Conditional Sterility
As effective and practical as steam sterilization is, it requires a porous barrier which creates inefficiencies and problems. The porous barrier allows for necessary steam movement but also creates a potential bacterial inroad. It is most commonly made of a paper-thin product composed of polypropylene, a plastic. By nature, the porous barrier is particularly sensitive to moisture and is physically fragile. The sterility of a porous barrier requires a carefully controlled environment and offers conditional sterility.
The cooling phase is a slow process due to the nature of the porous barrier and creates inefficient use of the autoclave. Steam must be allowed to gradually and naturally escape without causing condensation on the porous barrier. This starts with opening the autoclave door and letting contents cool within the autoclave. Afterwards, contents are pulled forward to sit in front of the autoclave until room temperature (Figure 9). The process cannot be forced and prevents the autoclave from being immediately reused.
An inappropriately fast cool time and early handling may cause condensation, bacterial wicking and compromise sterility. If hot sterilized trays are too rapidly cooled or placed onto cold metal racks, droplet condensation can form on the body of the hot sterilized tray. This can compromise the sterilized tray with bacterial wicking and potentially its neighbor trays with focal wet spots and dripping (Figure 10), (Association of PeriOperative Registered Nurses 2019; American National Standards Institute Inc. and Association for the Advancement of Medical Instrumentation 2017).
The long cooling process can be in conflict with the rapid turnover demands of a busy operating room. Short cuts or inexperienced staff can inadvertently compromise sterility. Outbreaks of increased surgical infections in clean surgeries occur with early handling or moist hand hygiene in sterile processing (Dancer et al. 2012). Surgical site infections complicate 2% of surgeries in the United States and can result in significant morbidity and mortality (Association of PeriOperative Registered Nurses 2019; Evans et al. 2011). The estimate average cost of a surgical site infection is approximately $13,300 to $35,400 in 2018 (Association of PeriOperative Registered Nurses 2019). Costs can be greater than $90,000 if associated with arthroplasty (Association of PeriOperative Registered Nurses 2019, Berrios-Torres 2017). 18- 33% of surgical site infections are orthopedic (Rashidifard et al. 2018; American National Standards Institute Inc. and Association for the Advancement of Medical Instrumentation 2017; Evans et al. 2011). Orthopedic cases can require a high number of large, heavy surgical trays. The porous barrier requires meticulous and careful treatment by knowledgeable staff. Personnel and practice changes can directly impact proper processing and infection risk.
Similarly, the porous barrier provides conditional sterility in storage. After appropriate cooling, trays are moved into separate low traffic areas away from cold air vents. The porous barrier is not an absolute microbial barrier and is dependent on a carefully controlled storage environment until surgical use. The storage area should have limited exposure to moisture, dust, direct sunlight, handling, temperature and humidity changes. Storage contamination can arise from handling, air movement, humidity changes, temperature changes, high traffic locations, dust, vermin and open shelving (American National Standards Institute Inc. and Association for the Advancement of Medical Instrumentation 2017; Association of PeriOperative Registered Nurses 2019).
The porous barrier is paper-thin to allow steam movement but is also relatively fragile and susceptible to physical damage from tears, holes, punctures and abrasions (Figure 11). The incidence of blue wrap holes may be 5-15% of processed trays (Ronchetti 2023). Some hospitals minimize contact with the porous barrier by moving blue wrapped trays on large cafeteria style plastic trays. Other centers routinely double wrap trays contents which in turn may require additional autoclave and cooling times. Blue wrapped trays must be physically handled carefully by knowledgeable staff.
Blue wrapped trays can only be fully checked for tears and holes at the time of surgery. If an unexpected hole is present, the tray and its contents cannot be used. If a critical tray is compromised, the surgery may be delayed or canceled creating costly inefficient use of the expensive OR. One minute of the OR has been referenced at $100/minute but can range from $36-113/minute; a delay of 1 hour costs $2,160-6,780 (Childers and Maggard-Gibbons 2018; Ronchetti 2023; Ting et al. 2012). Blue wrap compromise is variable over time and center and is highly dependent on manual practices and labor. A change in sterile processing staff and practices can significantly affect compromised blue wrap incidence, detection, and infection risk (Mobley and Jackson 2018; Waked et al. 2007; Dancer et al. 2012).
Unfortunately, every surgeon and OR has experienced a blue wrap hole that has affected surgery. This is a costly delay in surgery that can affect patient outcomes. Notably, the intraoperative blue wrap inspection is subjective dependent on the evaluator (Figure 12). A recent study found blue wrap holes and tears that were intentionally created were detected only 56% of the time (Mobley and Jackson 2018). The study suggests the porous barrier may be an under-appreciated source of potential surgical infection (Waked et al. 2007; Mobley and Jackson 2018). Rigid containers with porous filters may be more susceptible to bacterial ingress, especially over time (Shaffer et al. 2015).
Notably, the autoclave sterilization process is repeatedly challenged and rigorously checked at multiple points through the cycle to ensure adequate sterilization. These include Bowie-Dick tests, chemical indicators, biological indicators. These frequent multimodal checks ensure adequate autoclave sterilization (Association of PeriOperative Registered Nurses 2019) (Figure 13).
Unlike the autoclave, the only full check on the porous barrier is subjective and at the time of surgery. This assumes the blue wrapped tray has been in a low traffic area that is humidity controlled and without exposure to any moisture droplets. This is not always the case as trays can be placed next to scrub sinks in high traffic hallways before surgeries (Figure 14). One drop of water or a moist hand may compromise the sterility of a blue wrapped tray. The autoclave sterilization process is checked multiple times with various methods; the porous barrier is fully checked only once, subjectively, right before its use.
A Digital Change to the Porous Barrier: A Verifiable Vacuum Seal
Without the porous barrier to allow steam movement, there can be no steam sterilization. As precise and rigorous steam sterilization in the autoclave has evolved with its multiple checks, the porous barrier has stayed relatively the same without innovation.
Novel electromagnetic container technology may offer a solution to the challenges of the porous barrier by removing the porous barrier altogether. The dry phase of the autoclave normally undergoes a negative pressure environment to remove steam. This sterile negative pressure phase can be harnessed to capture a sterile vacuum seal within a container in the autoclave. If a sterile vacuum seal can be captured and maintained within a container, no porous barrier will be required to maintain sterility outside the autoclave.
Zuno Medical has created a novel sterilization container using electronic technology to create a verifiable vacuum sealed container. It has received FDA de novo approval under a new category - rigid sterilization container with electronic monitoring. The novel container possesses two large electromagnetic valves and a controlling computer processer in the lid of the container. Digital electronic sensors in the interior of the lid of the container monitor temperature, pressure and moisture which inform the digital processor. The electronic components are powered by a standard lithium battery and are secured in proprietary housing to repeatedly withstand the harsh autoclave environment. Repeated electronic survivorship was an essential part of the de novo FDA approval.
The valves of the electronic container are open during the conditioning and exposure phases freely allowing the unimpeded flow of steam into the containers within the autoclave. After appropriate exposure and during the terminal negative pressure dry phase within the autoclave, magnetic valves are activated capturing the autoclave’s sterile environment within the electronic container. A subatmospheric vacuum seal (absolute 6-8 PSI) is contained and maintained by a specialized container body to withstand the vacuum forces.
The sterile environment created by the autoclave is captured and the container vacuum seal is maintained and monitored by the Zuno container. The vacuum seal replaces the porous barrier (Figure 15). The internal lid sensors continually detect an intact vacuum seal with real-time electronic monitoring providing a verifiable vacuum seal. The seal integrity can be confirmed at any time with a button push. Electronic technology allows for a digital verifiable vacuum seal or smart sterilization. When the smart container is opened, contents will be dry given the seal is created in the terminal dry phase of the autoclave.
The mechanics of the smart container and a walk through of the sterile process are illustrated with this short video - https://www.youtube.com/watch?v=Rpx1gwy9qHY . Digital container technology allows the sterile autoclave environment to continue outside the autoclave until it reaches the OR under a verifiable vacuum seal.
Once the autoclave cycle is completed and the door is open, the vacuum sealed container (VSC) can be handled with thermal protective gloves without theoretical risk of contamination since there is no porous barrier. The tray will be hot and should be handled with care, but there is no risk of microorganism contamination with moisture wicking. This removes the cooling phase altogether and frees up the autoclave for immediate use increasing efficiency. The traditional cooling phase, typically 30 minutes - 2 hours or more for each cycle, may no longer be necessary with a VSC.
Sterile processing and operating room efficiency are improved by reducing porous barrier requirements. Increasing processing scale and reducing porous barrier amounts has been shown to reduce first case delays by 15 minutes and reduce OR turnover time by 31%, OR set up time by 94%, sterile processing manhours by 51% and surgical waste by 50-60% (Bradley et al. 2021). These efficiencies may be even greater by forgoing the porous barrier altogether with VSC technology.
Unlike a porous barrier, a VSC may be less susceptible to sterile compromise by environmental changes and physical handling. Humidity changes, water splashes, cold air vents, foot traffic, moist touching, early handling, damage with minor physical handling which would otherwise cause tears and holes in blue wrap, pose less of a threat to a VSC. A drop of water on a VSC will not compromise sterility like it does with a blue wrapped tray.
The VSC may be less susceptible to human error. The porous barrier requires knowledgeable, experienced staff with careful and meticulous handling and storage in a carefully controlled environment. Early handling leads to outbreaks of orthopedic surgical infections (Dancer et al. 2012). A VSC does not require proper blue paper wrapping, filter changes, waiting for natural cooling, no touching during a long cooling phase, thermal guns, and careful storage. In a high tray, rapid turnover busy operating room environment, sterile processing staff are under pressure to perform at a high level rapidly. A VSC may reduce the pressure on processing staff by foregoing a porous barrier, removing the cooling phase, and providing a verifiable vacuum seal.
With real time electronic monitoring, the integrity of the seal can be verified at any time instantaneously with a button push. A green check mark will illuminate confirming an intact seal. A red X will illuminate if the seal is not intact (Figure 16). This electronic check can be performed at any time after autoclave processing. A physical check can also be performed at any point as well by confirming the valves are in the upright and sealed position. These checks can be performed by the sterile processing staff without breaking the sterile barrier at any point - right after autoclave sterilization, the night before surgery, the morning before surgery, and in the OR by the circulating nurse or surgical technician. A verifiable VSC may help avoid the last minute OR delay or cancellation by foregoing the dreaded subjective blue wrap hole.
By reducing human error, physical damage risk and environmental compromise, VSC technology may lower peri-operative infections. The porous barrier may be an underappreciated source of infection (Waked et al. 2007; Mobley and Jackson 2018; Shaffer et al. 2015; Dancer et al. 2012) and may dramatically reduce morbidity, mortality, hospitalization costs associated with peri-operative infections.
Several centers have centralized sterile processing services and transport their sterilized trays to different surgical facilities in plastic bags and ground transportation (Nadeau, n.d.). The physical transport can still be precarious for paper-thin blue wrapped trays and must be handled carefully in humidity and temperature-controlled vehicles (Nadeau, n.d.). A VSC can potentially be transported between centers more easily and may also help forego the necessity of repeat sterilization between different sites. The integrity of the VSC can be verified throughout the transport cycle.
A VSC may also be more sustainable, environmentally conscientious and cost effective (Figure 17). It does not require traditional plastic disposables. Porous barriers are made of polypropylene, plastic #5. Plastic blue wrap composes 19% of operating room waste or 5% of total hospital waste (Reusable Totes, Blue Wrap Recycling and Composting, Environmental Best Practices For Health Care Facilities, JCAHO Environment of Care Standards 1.3, 2.3, 4.0, Prepared by Environmental Protection Agency 2022; Atlantic Health System 2019; Healthtrust 2017). It is estimated that 255 million pounds of blue wrap is thrown into landfills every year (Atlantic Health System 2019; Healthtrust 2017) (Figure 13). Reducing 1 million pounds of blue wrap is equivalent to taking 250 cars off the road. (Atlantic Health System 2019; Healthtrust 2017) Blue wrap is not reusable according to the EPA (Reusable Totes, Blue Wrap Recycling and Composting, Environmental Best Practices For Health Care Facilities, JCAHO Environment of Care Standards 1.3, 2.3, 4.0, Prepared by Environmental Protection Agency 2022). Blue wrap processed as biowaste is costly and cost $20,000 annually for a surgical center (Trunick 2011). One hospital spent $217,285.36 on blue wrap in one year or 67,600 lbs of blue wrap annually (Babu et al. 2018). The reusable VSC foregoes expensive plastic problems with a sustainable and potentially cost-effective subscription model. Zuno personnel maintain and service the VSC’s while in use.
Conclusion
Steam sterilization has evolved after 2000 years. It is a practical, affordable, effective, highly regulated process. However, it requires a porous barrier that is paper-thin and fragile and requires a slow and inefficient cooling process. It is particularly sensitive to physical damage and moisture and requires careful environmental conditions. A compromise of the porous barrier can lead to costly OR delays or cancellations since it is checked subjectively right before its use in the OR. It may also be an overlooked source of perioperative infections. The porous barrier is an underappreciated weak point.
The negative pressure environment in the normal autoclave cycle of the sterilization process can be harnessed to capture a vacuum seal in the sterilized containers with electronic technology. This requires a novel digital electromagnetic container that foregoes the porous barrier and its limitations. A digital vacuum sealed container may be more monitorable and transportable and create a more resilient barrier to physical damage or moisture insults over blue wrap. A vacuum sealed container that is reuseable may offer a more cost effective, efficient and sustainable sterilization alternative allowing for digital improvement in patient care.