Introduction
The alignment of total knee arthroplasty (TKA) implants plays a significant role in implant survivorship and functionality (Sharkey et al. 2014; Sikorski 2008; Fang, Ritter, and Davis 2009; Liu et al. 2016). Malpositioning can lead to instability, pain, decreased range of motion, and implant loosening (Kumar and Dorr 1997; Hofmann et al. 2003; Sharkey et al. 2014; Bellemans et al. 2002). Computer-assisted technology was designed to improve the accuracy and reproducibility of implant positioning with higher precision of bony cuts and improved assessment of soft tissue tension. The first computer-assisted TKA navigation was performed in 1997 which ushered in a new method of performing knee arthroplasty (Leitner et al. 1997; Delp et al. 1998).
The adoption of computer-assisted surgery (CAS) for total knee navigation has been steadily increasing. The Australian Orthopaedic Association National Joint Replacement Registry reported that CAS TKA have risen from 2.4% to 32% since 2003 and 2019 respectively (“Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2020 Annual Report” 2020). Friederich et al. conducted a survey among 389 Swiss orthopaedic surgeons from 2006-2007 and found that 25.7% utilized a CAS when performing TKA in at least 75% of cases (Friederich and Verdonk 2008). Conversely, the adoption of navigated TKA in the United States has increased at a much slower rate when compared to other countries. Antonios et al. examined the Nationwide Inpatient Sample and found that from 2005 to 2014, the use of navigation in TKA procedures had increased from 1.2% to 6.3% in the United States (Antonios et al. 2019).
Navigation has been shown to improve overall implant positioning compared to conventional TKA techniques (Haaker et al. 2005; Anderson, Buehler, and Markel 2005; Chin et al. 2005). Although a majority of current navigation and robotic platforms are image-less, there are disadvantages when compared to image-based platforms.
Operative times with CAS are increased by 23% when compared to conventional techniques with one study comparing 108 patients finding that a mean of eleven minutes was required to initially setup and register the navigation (Dutton et al. 2008; Bauwens et al. 2007). This increased time was in-part due to a pointer probe which was used to register the bone morphology for the navigation system.
This increase in operative time is mainly related to the registration phase, in both image-based and image-less systems. The image-based systems are more accurate for detection of anatomical landmarks pre-operatively based on surface topography (bony or cartilage). They also provide the opportunity for surgeons to “pre-plan” the implant positioning prior to surgery to save time intra-operatively according to the overall alignment, thickness of bony resection, and implant sizing (Bae and Song 2011). The downside is that an MRI or CT scan is required, which adds cost or risk of radiation exposure.
In image-less systems, since there are no prior advanced images available, the surgeon is required to either “paint” the articulating surface with a pointer probe to obtain a more accurate joint surface and a complete anatomical surface, or manually choose the most important anatomical landmarks, which may not be consistently accurate (Jenny and Boeri 2004; Robinson et al. 2006). Manual use of pointer probes introduce user error and variability when recording bone morphology. Stulberg et al. demonstrated inaccuracies when registering a navigation system with a handheld pointer and demonstrated up to 12o of variability in the coronal plane and attributed the variability to knee morphology, software inaccuracy, and surgeon technique (Stulberg, Loan, and Sarin 2002).
A new technology utilizing laser scanning offers a quick and accurate method of acquiring bone morphology and does not rely on costly preoperative advanced imaging or additional time to paint the joint surface during surgery (Joshi and Rowe 2017). Our study sought to examine the utility of an intraoperative handheld laser scanner to acquire anatomical landmarks of the tibio-femoral joint. We hypothesize that the use of a laser scanner will allow for efficient registration and provide an accurate model of the tibio-femoral joint in a few seconds when compared to preoperative magnetic resonance imaging (MRI).
Methods
Specimen preparation and MRI imaging
This study involved cadaveric specimens which did not require institutional review board approval. The study included six fresh frozen cadaver knee joint specimens with native tibiofemoral joints. The specimens were initially imaged with a high-resolution MRI knee scan using a dedicated coil. This was combined with a low-resolution series of the lower lib with imaging of the Ankle, Knee and Hip. The images were segmented to produce 3-dimentional (3D) model of the articulating surface of the tibia and femur of each specimen using commercially available software (MIMICS, Materialise).
Surgical procedure and Laser Scan
Each specimen was placed in the supine position and secured to the table proximally. A leg holder was positioned to hold the knee at 90o of flexion. A standard medial parapatellar incision was carried out on each specimen. Soft tissue dissection was completed to allow for exposure of the tibia and femur. Care was taken to ensure that the articular surface was not damaged during the preparation. Once adequate exposure of the tibia and femur was achieved, a portable handheld laser scanner (E4D Technologies, Dallas, TX) was used to scan the surface topology of the cartilage (Figure 1). The femur and tibia were scanned separately. Each specimen was scanned 3 times by four different operators to calculate inter- and intra-observer reliability. Operators included a senior orthopedic surgeon and a fellow specialized in arthroplasty, as well as 2 engineers experienced in navigation technologies. Time for each scan was recorded.
Laser Scan Registration Validation
After the completion of laser scanning, the 3D models were registered to the segmented MRI scans that were completed prior to the procedure. An Iterative Closest Point (ICP) algorithm was used to align the handheld laser scans to the MRI scans within the registration application (Caira Surgical, New York, NY) (Figure 2). The accuracy of the registration fit was determined by the Root Mean Square (RMS) difference between the two surfaces in the region of interest. The ICP algorithm attempted to identify the rigid transformation that best fits a cloud of points with a model (Du et al. 2017). The algorithm uses a least-squares method to minimize the sum of the squared differences between both sets of points. The algorithm was preset to use 85% of the point cloud for matching to allow for surface variation and outliers.
Results
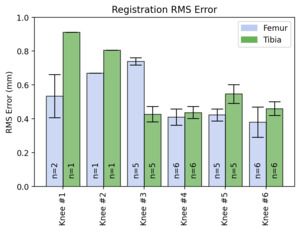
A total of 72 scans were completed by four different operators on the 6 cadaveric specimens. All scans were successfully registered and validated with the use of the ICP algorithm on the pre-procedure MRI. Each scan had an RMS error of <1mm for both the femoral and tibial scans (Figure 3). The mean time for successful registration with the handheld laser scanner was 54 ± 24 seconds. The inter- and intra-observer reliability were 0.92 and 0.82, respectively.
Discussion
The main goal of this study was to assess the utility of a handheld laser scanner for intraoperative registration of the tibial and femoral articular topography. This goal was achieved by comparing the 3D model formed by the laser scanner to models segmented from MRI scans collected prior to dissection of the cadaveric specimens. The results from our study demonstrated that surfaces of the tibia and femur could successfully be scanned using a handheld laser. When comparing the 3D models generated from the handheld laser scanner to the segmented MRI scans, the model had an average of less than 1 mm difference in RMS when compared to the scan. This was similarly seen by Chan et al. who utilized a light scanner to record bone topography and had an RMS error of 1.0 – 1.2mm in their in vitro study (Chan et al. 2016). However, the study utilized CT scan for segmented models to compare with the 3D scans, negating the cartilage. The current study was able to use MRI models, allowing for visualization of the cartilage.
Initial cost, user variability, need for specialized training, and increased operative time have hindered the adoption of CAS when performing a total knee arthroplasty in the United States (Jones and Jerabek 2018; Dutton et al. 2008; Bauwens et al. 2007). The use of a handheld laser scanner is able to overcome some of these limitations seen in other navigation systems. The laser scanner used in this study was able to achieve a highly accurate and reproducible registration of the joint surface topography with a mean time of 54 seconds. This is in contrast to the 5 to 11 minutes that may be necessary for probe-based navigation systems demonstrated in a study by Dutton et al (Dutton et al. 2008). Moreover, these results are based on a single surgeon performing 108 TKA which demonstrates the amount of time needed to register probe-based navigation systems even with experienced surgeons. Multiple operators in our study were able to accurately scan the tibio-femoral bone topography with minimal training and experience. Other navigation systems can require up to ten procedures for surgeons to become comfortable enough to acquire reproducible registration (Stulberg, Loan, and Sarin 2002). Similarly, robotics within other fields such as spine surgery may benefit from utilizing a laser scanner to reduce the registration time. Kostrzewski et al. utilized cadaveric models to demonstrate that registration and verification of takes approximately 7 minutes and 54 seconds to complete for cervical spine surgery (Kostrzewski et al. 2012).
The accuracy and reproducibility of measurements in a navigation system are crucial to ensuring appropriate positioning of TKA implants. Previous authors have discussed that deviations in coronal alignment of greater than 3° lead to increased risks of failure of the implants in a TKA (Berend et al. 2004; Ritter et al. 1994). The accuracy offered by laser scanners for TKA could help to reduce the risk of implant malpositioning. Winemaker et al. demonstrated that patients undergoing TKA with large preoperative alignment deformities averaging 17.1o had asymmetric flexion or extension gaps ≥3 mm when compared with patients with less preoperative deformity (Winemaker 2002). This asymmetry placed patients at increased risk of instability. The laser scanning technology assessed in this study is able to produce 3D models with a less than 1mm RMS when compared to MRI models. This accuracy may provide improvements in gap balancing especially in patients with larger deformities.
As this technology continues to improve, the authors believe that it will become integrated with the current navigation and robotics technology utilized in arthroplasty today. Rather than relying or CT scans or manual probing for knee surface topography for robotics, we hope to see this technology used to capture knee topography in real time during the procedure. Moreover, as technology continues to advance, we envision the ability to determine rotation utilizing a fiducial for augments 6 degrees of freedom tracking (Meftah 2022) based on bony anatomy similar to manual probing used in current navigation technology. The information gathered from the laser scanner will be combined with other information including standing alignment film radiographs to provide surgeons with the necessary information to accurately make their bony cuts and appropriate position implants.
There are several limitations to this study. Similar to other cadaveric studies, a limited number of specimens were tested in our study, however we had a very high inter- and intra-observer reliability. Lack of soft tissue bleeding was an additional limitation to this study, although scanning femur or tibia within 30 seconds can reduce the risk of blood interfering with registration. Additionally, preservation methods utilized for the cadavers may affect the MRI model, even though the cadavers were fresh-frozen.
In conclusion, the results of this cadaveric study demonstrated that a handheld laser scanner is a feasible method for registering the tibio-femoral joint. The technology is able to provide accurate, reproducible bone topography in a fraction of the time of other registration methods. These results demonstrate the utility of this technology when performing navigated TKA. Future studies should focus on assessing the technology through in vivo testing and the accuracy of implant positioning when compared to other navigation modalities. In addition, future studies should examine the integration of the laser registration with radar tracking technology to orient the scan with the surgical coordinate system.



_handheld_sc.png)


_handheld_sc.png)
