Introduction
The term “claw hand” was originally associated with Augusta Dejerine-Klumpke, reflecting the characteristic appearance of forearm supination, wrist and metacarpophalangeal (MCP) joint extension, and finger flexion she observed in lower brachial plexus injuries (Ulgen et al. 2008; Shoja and Tubbs 2007). However, today “claw hand” is broadly used to describe the resultant appearance of a spectrum of conditions. Clawing itself is defined as the posture of MCP hyperextension and proximal interphalangeal (PIP) joint flexion (Bauer and Chaise 2022). It can be secondary to chronic compressive neuropathy such as cubital tunnel syndrome, traumatic nerve injury, burn contracture, compartment syndrome, peripheral neuropathy such as Charcot-Marie-Tooth, infectious sequelae such as Hansen’s disease, congenital contractures of the intrinsic muscles or volar plate, neuromuscular disorders, cervical spondylosis, and cerebral infarct (Deguchi et al. 2021; Anderson 2006; Fufa, Chuang, and Yang 2013; Vinci et al. 2005; Neiman, Maiocco, and Deeney 1998; Baron and Strohl 2020; Kumar and Chandraprakasam 2011; Muellbacher et al. 2002; Jiravichitchai et al. 2021). The extent of the clawed appearance and severity of functional deficits are similarly variable depending on the inciting event, number of fingers affected, chronicity, and attempts at maintaining supple joint motion.
Various treatment options have been proposed to correct the clawed appearance, restore synchronous finger flexion, and enhance gripping ability (Brandsma and Brand 1992). Surgical interventions are broadly categorized as static or dynamic depending on the chronicity and extent of clawing (Starr and Chung 2022). These procedures have been repeatedly modified over the years and include capsulodesis, tenodesis, bone blocks, and tendon transfers (Hastings and Davidson 1988; Sapienza and Green 2012; Gupta, Consul, and Swamy 2015; Özkan, Özer, and Gülgönen 2003; Taylor et al. 2004; Ratner, Peljovich, and Kozin 2010). Additional variability is present when one considers the number of fingers to be corrected, the need for autograft tendon, dorsal versus volar vector for tendon transfer, number of potential donor muscles for transfer, and differing distal insertion sites. Furthermore, certain underlying conditions, such as coexistent ulnar and median neuropathy or stroke, may preclude many of the surgical options that rely on median-innervated myotendinous donors (Brandsma and Brand 1992; Sapienza and Green 2012). Although multiple descriptions and schematics exist summarizing various procedures, the techniques themselves are often presented in cursory detail as part of topic reviews (Sapienza and Green 2012). In particular, there is a paucity of complete, step-wise, and reproducible technique to perform claw correction in patients after cerebral infarct. These patients typically suffer from complex clawing of all four fingers, may have additional limitation of thumb function, and require strong grasp to use ambulatory aids given the lower extremity deficits that commonly follow a stroke.
The present work utilizes the case of a 43-year-old male with clawing secondary to stroke to highlight pre-operative assessment, expectation management, and technique of claw correction. The surgical technique for four finger complex claw correction is described via all dorsal extensor carpi radialis brevis (ECRB) tendon transfer to the lateral bands, a modification of the original Brand procedure (Brand 1961). Four-tailed plantaris tendon autograft harvest and preparation are described in step-wise fashion. The technique improves the clawed appearance, restores synchrony of MCP and PIP joints during flexion, and enhances grip strength, without crossing through the carpal tunnel or relying on median-innervated motor units.
Pre-Operative Assessment
In chronic clawing, restoration of “normal” hand appearance and function is rarely, if ever, achieved and is not the goal. Rather, the goal is to improve a patient’s ability for meaningful use of their hand by augmenting strength and restoring more synchronous motion to improve dexterity. It is imperative to ascertain the patient’s particular limitations and tasks they find difficult. A common complaint and challenge is the inability to adequately wrap the fingers around objects during attempted grip. Establishing mutual understanding of specific limitations guides expectations and surgical decision making. Claw correction can reliably restore functional grasping ability, but is less reliable in restoring high dexterity movements, such as playing musical instruments. The surgeon and patient must embark on this path with shared understanding about achievable goals.
The etiology of clawing should be determined. This ensures the patient receives appropriate medical management for underlying conditions or associated spasticity. Duration of symptoms and prior treatments are assessed, including therapy, static and/or dynamic splinting, and previous operations. One must ensure that the patient has maximized improvement with therapy for joint motion, and that functional impairments persist, prior to making a decision for surgery. Information regarding previous surgery is essential to know if bone block procedures have been attempted, and what donor myotendinous units are still available.
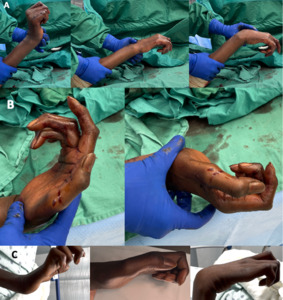
Physical examination assesses the gross appearance of the clawed hand, focusing on the relative involvement of all the fingers, presence of atrophy, and surgical scars (Figure 1A). Passive and active range of motion of the wrist and individual finger joints are assessed to gauge for capsular contracture and extensor lag from central slip attenuation. Ability to actively open and close the hand, grasp objects, perform thumb-to-index finger pinch, perform key pinch, and manipulate basic objects is evaluated (Figure 1B). The pattern of finger closing is observed for loss of MCP and PIP joint synchrony. This creates a rolling motion, restricting the fingers from forming around larger objects during grip. Patients may exhibit compensatory wrist flexion during attempted grip in an effort to increase finger extension, known as the Andre-Thomas sign. Depending on the extent of neurologic deficits, thumb pinch and/or opposition function may be affected as well (Figure 1C). Thumb deficits are not reconstructed in the same stage, as claw correction often restores hand function such that patients are satisfied and do not wish to pursue further adductorplasty or opponensplasty. These options certainly are reasonable at a later stage.
One must determine if clawing is simple or complex, as this informs the surgeon regarding static versus dynamic procedures, respectively. The Bouvier test determines simple versus complex clawing (Figure 2A). Then, one must determine if complex clawing is solely due to central slip attenuation, or the additional presence of extrinsic flexor myotendinous tightness and/or intrinsic contracture (Figure 2B). Flexor myotendinous contracture can be address with Z-lengthening at the tendon level (for wrist flexors) or fractional lengthening at the myotendinous junction (for finger flexors). Of note, addressing tight wrist flexors carries the additional benefit of augmenting wrist extension, while addressing tight finger flexors may in fact weaken grip strength. It is important to discuss potential pros and cons with the patient, as the desire for strong grip may supersede a small amount of residual PIP joint flexion. Intrinsic contracture may involve the capsule, volar plate, and/or collateral ligaments, and is addressed via sequential release.
Plain hand radiographs should be obtained to assess for joint subluxation or dislocation, degenerative changes, and evidence of prior attempts at surgical claw correction with bone block or arthrodesis (Figure 3). Ultrasound of bilateral forearms and legs is utilized to ensure the patient has palmaris longus and plantaris tendons, particularly in settings of prior surgery. Plantaris is preferred for extensor-based four tail claw correction, as palmaris longus is often too short. If bilateral plantaris are absent, allograft can be used. Finally, electrodiagnostic studies are obtained to confirm the presence of muscle denervation changes and lack of evidence of reinnervation. They can further differentiate localized nerve entrapment and mono- or polyneuropathy in cases where the etiology of clawing is uncertain (Gooch and Weimer 2007).
Patient Presentation
This is a 43-year-old male who suffered a cerebral infarct twenty years prior, resulting in clawing of bilateral hands. He presented seeking to improve hand function, focusing on the right hand as it was his dominant side. Particularly, he expressed inability to grasp large objects, and a weak and clumsy grip with smaller objects. For instance, he could not hold a drinking glass with a single hand, experienced difficulty holding his young children and performing their hygiene, and had routine challenge in his job as a field operator. He had not undergone prior surgery, and was regular with therapy to preserve hand function and motion. In discussion, he was very realistic and aware that “normal” appearance and function could not be restored, and simply desired better ability to grasp larger objects.
The patient’s hand appearance, motion, and function are depicted in Figure 1. Clawing affected all four fingers, more pronounced in the ring and small. Thumb function was also impaired, with thenar atrophy, lack of opposition, and weak key pinch exhibiting Froment’s sign. This presentation mimicking dual median and ulnar nerve deficits is not uncommon in patients suffering a stroke. The patient did not have spasticity, which was reassuring, as spasticity may compromise volitional control of dynamic transfers. The patient’s Bouvier test confirmed complex clawing secondary to central slip attenuation (Figure 2A). Further extrinsic flexor myotendinous tightness was present (Figure 2B), limiting both PIP extension and wrist extension. Plain radiographs (Figure 3) demonstrated no degenerative joint changes or evidence of prior surgery. Ultrasound showed absent palmaris longus bilaterally, but both plantaris tendons were present. Electrodiagnostic studies did not reveal concomitant peripheral neuropathy.
Treatment goals included improving the ability to grasp and enhancing grip strength. A dynamic tendon transfer can accomplish these by yoking MCP flexion and IP joint extension, allowing synchronous wrapping of the fingers around larger objects. Addressing tight wrist flexors (flexor carpi radialis and flexor carpi ulnaris) via Z-lengthening was recommended, as improving wrist extension motion simultaneously augments natural tenodesis for additional grip strength. Tight finger flexors were not addressed, as the patient did not wish to compromise ultimate grip strength. Finally, addressing thumb opposition and key pinch deficits was not recommended in a single stage, and can be reassessed following recovery from initial claw correction if desired.
As shown in Table 1, multiple dynamic transfer options exist to address complex clawing. All-dorsal ECRB tendon transfer to the lateral bands via plantaris autograft was chosen. Given the combined deficits to median- and ulnar-innervated muscles secondary to cerebral infarct, a wrist extensor was chosen as the motor to power the correction. As the central wrist extensor, ECRB was selected to maintain balanced wrist extension by sparing ECRL and ECU. Dorsal route was chosen rather than volar to permit ease of tendon transfer and tensioning the four fingers. Furthermore, it allowed ECRB transposition superficial to the extensor retinaculum to minimize adhesions and create mild bowstringing, further augmenting extension power. Plantaris autograft was utilized due to adequate length for four-tailed graft, thin and flat profile similar to the extensor tendons, and decreased cost compared to allograft.
Plantaris Autograft Harvest
The patient is positioned supine, a tourniquet is placed on the thigh, and the operative leg is externally rotated for medial access. Palpable landmarks are the medial malleolus, calcaneus, and Achilles tendon. Plantaris inserts on the superior aspect of the posteromedial calcaneus, just anterior to the Achilles tendon insertion. As it courses proximally, the tendon progresses from a medial to a lateral position towards its origin at the lateral supracondylar line of the femur (Gonera et al. 2021). This gradual medial-to-lateral transition must be kept in mind during the proximal harvest.
The initial longitudinal incision is two finger breadths posterior to the medial malleolus and 2-3cm proximal to the superior border of the calcaneus, slightly anterior to the palpable Achilles tendon. Dissection through subcutaneous and fatty tissue readily exposes the Achilles tendon (Figure 4A). Plantaris is found just anteriorly, confirmed by pulling on the tendon to elicit weak plantarflexion and inversion. The posterior tibial artery and tibial nerve are further anterior and deeper, between the flexor digitorum longus and flexor hallucis longus, and are not at risk during the dissection.
Plantaris is then harvested via a series of small incisions progressing proximally along the leg. An encircling tendon grasper, such as an Alice clamp, is used to pull on plantaris as one palpates 4-5cm proximally on the leg for ballottement. Another incision is made at this level and blunt dissection is used to locate plantaris, aided by intermittently pulling on the tendon in the distal incision. Once located in the more proximal incision, it is similarly grasped and pulled to determine the location for the next proximal incision. This continues until adequate tendon length is achieved, typically via 4-5 total incisions (Figure 4B). As mentioned, plantaris courses further laterally as one progresses proximally in the leg, and is located deep to the gastrocnemius and superficial to the soleus (Figure 4C).
Once adequate length is obtained, the plantaris tendon is cut proximally, sequentially delivered into the distal incisions, and ultimately released off the calcaneal insertion. One can routinely obtain 30cm or greater tendon length via this technique (Figure 4D). Harvest incisions are irrigated, tourniquet is released, and wounds are closed in standard fashion. The autograft is wrapped in a moist sponge as attention is turned to the arm.
Extensor Carpi Radialis Brevis Harvest & Transposition
The patient remains supine with operative arm pronated on a hand table and tourniquet placed about the upper arm. A 3cm longitudinal incision is made over the base of the third metacarpal dorsally (Figure 5A). The ECRB insertion is located and fully released subperiosteally from the third metacarpal, taking care to protect the nearby extensor digitorum communis (EDC) tendon. A tagging suture is placed into ECRB for ease of handling.
A second longitudinal incision is made 3cm proximal to Lister’s tubercle (Figure 5A). This ensures that one is proximal to the extensor retinaculum, which can be of variable length, from 1.5-3.5cm. ECRB is located here within the second extensor compartment without violating the extensor retinaculum. Areolar adhesions to ECRL are released and ECRB is delivered proximally into this incision. To avoid damaging the tendon, a hemostat is passed antegrade through the proximal incision deep to the extensor retinaculum, the tagging suture placed earlier is grasped, and retrieved retrograde to deliver ECRB. The ECRB will again be passed into the distal wrist incision, but superficial to the extensor retinaculum through a subcutaneous tunnel. Transposing ECRB superficial to the retinaculum creates mild bowstringing, strengthening its wrist extension moment. The superficial position further facilitates ease of eventual tendon weaving with the plantaris autograft, and decreases the risk of adhesions if ECRB is maintained deep to the retinaculum (Sammer and Chung 2009).
Lateral Band Identification
A small bolster is placed under the hand and fingers, and incisions are marked over the lateral bands of the index, middle, ring, and small fingers at the level of the proximal phalanges. Incisions are dorsal to the mid-axis of the fingers, with a slight dorsal-distal and volar-proximal trajectory, akin to the lateral bands themselves (Figure 5B). For the middle, ring, and small fingers, the incisions are centered over the radial lateral bands to prevent undesired ulnar drift of the fingers after tendon transfer (Figure 5B). However, the index finger incision is centered over the ulnar lateral band for two reasons: 1) prevent undesired resting abduction posture of the index finger, as this position can make the thumb miss the index finger during attempted pinch grip, and 2) add a supination moment to the index finger, augmenting stability during pinch grip.
Dissection is carried through the finger incisions until the extensor expansion is seen (Figure 5C). The distinction between the central slip and lateral band can be identified based on fiber direction (longitudinal fibers of the central slip and oblique fibers of the lateral band). A scalpel is used to sharply separate this plane between the central slip and lateral band fibers for eventual tendon transfer. Care is taken to only separate this plane over the proximal phalanx for eventual tendon weaving, while preserving the distal lateral band insertion.
Plantaris Autograft Preparation
The autograft is equally looped into a “U” shape and each end is sharply divided in two, creating four total tails (Figure 6A). Estimating appropriate length for the tails can be challenging. Tails that are too short will not evenly reach all four lateral bands for appropriate excursion after tendon transfer. Tails that are too long will be very thin proximally over the carpus and make tendon weaving with ECRB challenging. Our preferred method is to measure the distance from the proximal aspect of the lateral bands in the finger incisions to the ECRB insertion in the distal wrist incision. Decrease this distance by 3cm to ensure the tendon transfer is tight at the time of inset, and to account for eventual creep. In this case the above distance measured 10cm, so 7cm length was used for each of the four autograft tails.
Tendon Transfer
Prepared plantaris autograft is laid over the dorsal hand with the four tails distally towards the fingers (Figure 6B). The graft should appear longer than necessary, as it shortens with multiple tendon passes and excess length can be trimmed following tendon weaving. A curved tendon passer is advanced retrograde through the finger incisions, ensuring to pass volar to the deep transverse metacarpal ligaments via the lumbrical canals and into the intermetacarpal spaces of the hand. This is crucial, as the graft must pass in this fashion through the lumbrical canals to mimic lateral band trajectory, creating flexion at the MCPs and extension at the PIPs. The tendon passer is advanced through the dorsal wrist incision, one tail of the plantaris autograft is grasped, and delivered antegrade into the respective finger incision. This is repeated for all four fingers and graft tails (Figure 6C).
A sharp tendon weaver is used to inset each plantaris autograft tail into the respective lateral band in a Pulvertaft weave (Figure 6D and E). In these cases, the digital extensor mechanism is attenuated and slightly elongated secondary to the chronically clawed posture. During the first tendon weave, an assistant uses a hemostat to grasp the proximal aspect of the lateral band and gently pull proximally. This eliminates redundancy in the lateral band during the first tendon inset weave. Typically two-to-three Pulvertaft passes of the autograft tail are performed for each lateral band. We prefer to use 4-0 non-absorbable braided suture (FiberWire, Arthrex, Florida, U.S.A.) for tendon inset. This process is repeated for each of the four lateral bands. Of note, one does not need to position the fingers or wrist in any particular manner during this portion. Final tensioning and balancing of the tendon transfer is performed proximally at the dorsal wrist incision, as this is easier than attempting to tension at the finger level.
After weaving the autograft tails into the four lateral bands, the wrist is placed in 45o extension, metacarpophalangeal joints in 90o flexion, and interphalangeal joints in full extension, mimicking intrinsic plus position. Placing the hand over a small irrigation basin or folded towels facilitates maintenance of this posture. The transposed ECRB now lies superficial to the extensor retinaculum within the distal wrist incision. The ECRB tagging suture is pulled distally to assess the location to weave the plantaris autograft. The sharp tendon weaver is passed through ECRB, the looped proximal end of the plantaris autograft is grasped and delivered through ECRB, and sutured in place. The challenge at this stage is typically balancing the correction for all four fingers, as some may be corrected and others still assuming a slightly clawed posture. One can individually tighten the plantaris tail to any finger as needed, simply by pulling proximally on the tail and suturing in a reefing manner through the distal wrist incision. Once the desired hand posture is achieved, continue the Pulvertaft weave of plantaris through ECRB for a total of three to four passes (Figure 6F).
Passive wrist extension and flexion are performed to assess tenodesis of finger flexion and extension, respectively (Figure 7A), and to assess the digital cascade (Figure 7B). Wounds are irrigated, tourniquet is released, and incisions are closed in standard fashion.
Post-Operative Protocol & Rehabilitation
A short arm plaster splint to maintain intrinsic plus position is placed at the conclusion of the procedure. This is replaced with a short arm cast in a similar intrinsic plus mold at the first post-operative visit, for a total of four weeks immobilization. From four to eight weeks, a removable thermoplastic splint in the same posture is used at all times except twice daily for gentle active finger range of motion and tendon transfer re-education under the guidance of hand therapists. No passive manipulation or strengthening is performed. From eight to twelve weeks, the thermoplastic splint is worn only at night time. Continued active motion, transfer re-education, and task-specific training are performed under hand therapist guidance. At twelve weeks, the thermoplastic splint is discontinued and gradual strengthening is begun.
Comparative images illustrate the pre-operative and post-operative appearance of the hand (Figure 7C). Assessment of hand opening, closing, and grip activity is demonstrated at three months post-op (Figure 8). The patient expressed great satisfaction with the correction, as he was now able to hold and manipulate household objects and assist with his children’s hygiene. His improved ability to grip allowed him to return to work as a field operator. Although he still had deficits of thumb opposition and key pinch, he was happy with the overall outcome and did not wish to pursue further surgery for the thumb.
Conclusion
Clawing refers to a wide spectrum of debilitation in both hand appearance and function, secondary to a variety of conditions. Therapy to maintain supple joints is paramount to prevent contracture development and maximize the ultimate surgical outcome. Prior to any operation, both surgeon and patient must establish mutual understanding on the particular tasks and activities that are difficult, and set expectations on what can be improved. The numerous surgical options are quite nuanced, depending on extent of clawing, chronicity, and involvement of additional nerves and myotendinous units. In cases where simultaneous median neuropathy exists, or in patients following a stroke, complex claw correction requires a wrist extensor to power the transfer. The present technique utilizing plantaris autograft and all dorsal ECRB tendon transfer via four tails to the lateral bands is an effective method to correct complex clawing in such patients.








_ecrb_harvest__2)_ecrb_transposition__an.png)










_ecrb_harvest__2)_ecrb_transposition__an.png)



