Introduction
The treatment of massive rotator cuff tears (MRCT) in young and active patients without advanced glenohumeral degeneration remains a challenging dilemma facing shoulder surgeons. MRCT make up 10-40% of all rotator cuff tears (Paulo 2022) and despite recent advancements in surgical repair techniques and instrumentation, retear rates range between 40%-90% (Aguado et al. 2019). Several strategies exist to address MRCT, including partial repair, interpositional grafts, superior capsule reconstruction (SCR), subacromial balloon spacers, and reverse total shoulder arthroplasty (RTSA). Despite the varied options for MRCT, there is no consensus on the preferred option or evidence-based literature to guide care. Meta-analyses have failed to identify any one treatment as superior and are confounded by heterogenous patient populations (Kovacevic et al. 2020; Kucirek, Hung, and Wong 2021). These procedures raise concerns for donor site morbidity, high implant/graft costs, complex techniques, and longer surgical times (Berthold et al. 2021a).
This article will review the utility of using the long head of the biceps tendon (LHBT), which is a locally available and biologically viable autograft, for anterior cable reconstruction (ACR) to augment massive MRCT repairs (Adrian and Field 2020; Kim et al. 2021).
Anatomy
The LHBT originates as an intra-articular structure at the supraglenoid tubercle of the scapula. It then passes over the humeral head before exiting the glenohumeral joint through the bicipital groove (Elser et al. 2011). The size of the tendon is variable; however, it is approximately 5 to 6 mm in diameter and 9 cm in length, with the intra-articular portion wide and flat compared to the more tubular and smaller extra-articular portion (Elser et al. 2011). The anterior humeral circumflex artery supplies the intra-articular portion of the LHBT; the more distal portion is fibrocartilaginous and avascular to allow it to slide within its sheath in the bicipital groove (Elser et al. 2011). There is also a concentrated “net-like” pattern of sensory and sympathetic innervations at the biceps anchor, which becomes more attenuated distally at the myotendinous junction (Alpantaki et al. 2005). A soft tissue sling stabilizes the extra-articular portion of the LHBT as it enters the bicipital groove. Histological studies have shown that this sling is comprised of fibers from the coracohumeral ligament, superior glenohumeral ligament, and the subscapularis tendon (Park et al. 2018). The LHBT also slides up to 18 mm in and out of the glenohumeral joint in forward flexion and internal rotation compared with a referenced neutral arm position with neutral rotation (Savarese and Romeo 2012). Even though the function of the LHBT has been studied extensively, its impact on glenohumeral kinematics remains poorly understood. However, cadaveric and in vivo biomechanical studies have suggested that it may contribute to glenohumeral stability (Pagnani et al. 1996; Itoi et al. 1993).
Indications
This ACR procedure ideally addresses the patient with pain and limitation from supraspinatus deficiency. Conceptually, the ACR is used in conjunction with rotator cuff repair and serves a biological and biomechanical role in enhancing the healing of the residual cuff.
Establishing the repairability of the rotator cuff is a challenge beyond the scope of this review. However, it includes acuity of injury, active and passive range of motion, clinical exam, X-ray, and MRI findings. The Rotator Cuff Healing Index (ROHI) can be utilized to aid with the decision-making process. It is a valuable tool that identifies risk factors for non-healing and integrates them into a scoring system that is weighted according to their odds ratios (Kwon et al. 2018). Patients with scores greater than five will be candidates for an augmentation procedure.
Much like other superior rotator cuff procedures, the patient should have no evidence of shoulder escape and at least 90 degrees of active forward flexion. The subscapularis should be intact or easily repairable with less than grade 2 Goutallier changes.
Similarly, the infraspinatus should be repairable with less than grade 3 changes. When the infraspinatus is irreparable or is severely atrophied, we favor a lower trapezius transfer. We have not quantified if the Hamada classification affects patient outcomes.
Finally, the long-head biceps needs to be intact. SLAP tears and degenerative tearing/changes are not contraindications for ACR.
Surgical Technique
For surgeons who routinely perform arthroscopic rotator cuff repair, ACR with the LHBT procedure is relatively straightforward. Patient positioning is left to the surgeon’s discretion; however, the author’s preferred choice is beach chair for rotator cuff pathology. A diagnostic arthroscopy is first performed through a standard posterior viewing portal. Intra-articular and subscapularis pathology are addressed at this time, and the undersurface of the rotator cuff is examined. If the tear is small and the LHBT is diseased, the surgeon can elect to perform their tenodesis of choice. However, if the tear is large, the LHBT is left intact on the supraglenoid tubercle (Fig. 1). SLAP tears or fraying may be lightly debrided but are certainly not a contraindication to ACR.
The arthroscope is then placed into the subacromial space from the posterolateral viewing portal, and an anterolateral working portal is made under direct visualization. The definitive decision to perform the procedure is made at this time after evaluating the integrity of the infraspinatus tissue and the repairability of the supraspinatus. We believe that the infraspinatus must be repairable or intact to get the optimal benefit from an ACR procedure. Preoperative MRI is a valuable tool in predicting infraspinatus repair, and advanced atrophy (greater than Goutallier 3) and gross weakness in external rotation may better be treated with lower trapezius transfer.
Once the supraspinatus is deemed insufficient or irreparable, the decision to proceed with ACR is made. The transverse humeral ligament and tissue along the lateral bicipital groove are released with electrocautery. Small vessels at the superior margin of the pectoralis tendon are frequently encountered and may serve as a useful marker to recognize the distal extent of dissection. The release must be adequate so that the LHBT can be readily subluxated laterally out of the groove and on top of the tuberosity. Where to position the LHBT on the tuberosity depends on the remaining rotator cuff tissue. If the supraspinatus is repairable but deemed insufficient and at high risk for recurrent failure, then the LHBT is attached immediately posterior to the bicipital groove and fixed as an augment to the undersurface of the supraspinatus.
If the supraspinatus is deficient and cannot be reattached to the tuberosity, attention is then turned to the posterior rotator cuff as the infraspinatus is mobilized entirely so that it can be fixed onto the greater tuberosity with anterior advancement. The infraspinatus is advanced to the degree that tissue is not placed under significant tension. The greater tuberosity is then gently excoriated with an arthroscopic burr to provide a healing surface. Care must be taken not to decorticate the greater tuberosity to allow for stronger suture anchor fixation. The infraspinatus tendon is repaired using suture anchors with tapes. The exact repair depends on the morphology of the tear. The most common repair pattern uses a triple-loaded anchor with suture tapes to create a broad-based repair. If possible, this can be linked to a lateral anchor, but the tear pattern usually does not accommodate this.
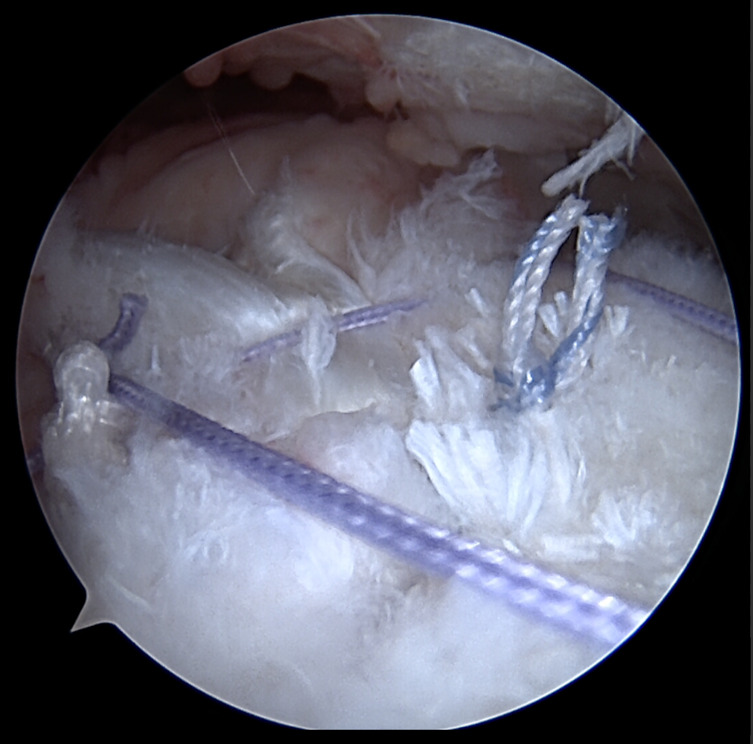
The LHBT is then repaired with two anchors. The first anchor is on the greater tuberosity in the middle of the supraspinatus footprint. The second, more distal anchor, is placed lateral to the bicipital groove and functions to pull the LHBT out of the groove. The first anchor (on the tuberosity) is a triple-loaded anchor, or a double-loaded knotless anchor, with tapes (Fig 2). The first suture is passed around the LHBT and tied into place. The second suture limb is passed through the LHBT and then into the infraspinatus in a side-to-side fashion (Fig 3). The third suture is placed around the LHBT and again into the infraspinatus, effectively completing the repair of the infraspinatus to the LHBT (Fig 5). The final anchor is placed at the same position where a suprapectoral arthroscopic tenodesis may occur, but just lateral to the bicipital groove. The transverse humeral ligament and soft tissues have already been debrided, allowing for tendon subluxation out of the groove.
The residual posterior rotator cuff may then be repaired to the biceps with side-to-side sutures to close any residual defect.
Biomechanics
Several biomechanical studies have examined the role of ACR in restoring joint mechanics in the setting of MRCT. In MRCT, loss of the concavity-compression mechanism leads to the unopposed pull of the deltoid and subsequent superior humeral head migration and acromial abutment (Cohn et al. 2022).
Park et al. examined these parameters in 9 cadaveric shoulders with type-II and type-III rotator cuff tears that underwent an ACR with an autologous LHBT. The cadaveric shoulders were tested at varying degrees of external rotation and abduction. They found that compared to shoulders with stage-III tears, shoulders with stage-II tears that underwent ACR with LHBT had significantly lower superior humeral head translation at 0°, 30°, and 60° of external rotation for both 0° and 20° of abduction. For stage-III tears that underwent ACR with LHBT, there was a significant decrease in superior humeral head translation at 0° and 30° of external rotation for 0° and 20° of abduction. Subacromial contact pressures were also significantly reduced for stage-III tears that underwent ACR with LHBT at 30° and 60° of external rotation with 0° and 40° of abduction and at 30° of external rotation with 20° of abduction.
Berthold et al. similarly examined superior humeral head migration and subacromial contact pressures, but in a dynamic biomechanical model with the shoulders abducted from 0° to 60° in neutral rotation. They performed an ACR with autologous LHBT using three different fixation techniques in the setting of irreparable posterosuperior rotator cuff tears. Their results demonstrated a significant reduction in superior head migration and subacromial contact pressures, regardless of fixation technique, when compared to controls.
These findings align with the results seen in the study by Krishnan et al., who biomechanically compared SCR using human dermal (HD) allograft and autologous LHBT in 8 cadaveric shoulders. The shoulders were tested under Five conditions: an intact rotator cuff, a complete supraspinatus tear, SCR with HD allograft with side-to-side sutures to the infraspinatus, and SCR with autologous LHBT with and without side-to-side sutures to the infraspinatus. Their results were consistent with previous studies in demonstrating a decrease in superior humeral head migration in shoulders that underwent SCR, irrespective of graft choice, compared to shoulders with complete supraspinatus tears. Superior humeral head migration was significantly lower at 30° and 60° of abduction but not at 90°. There was no statistical difference in superior humeral head migration between the HD allograft and LHBT autograft techniques.
Biomechanical models using allografts to reconstruct the anterior cable have also been shown to improve superior glenohumeral stability in the setting of MRCT. Denard et al. used a V-shaped semitendinosus allograft for anterior cable reconstruction. They found that superior humeral head translation was significantly decreased at 0° and 20° of abduction with 0°, 30°, and 60° of external rotation. There was also a significant decrease at 40° of abduction and 0° of external rotation. Regarding subacromial contact pressures, the V-shaped allograft restored peak pressure to intact levels for all positions except 40° of abduction and 60° of humeral head rotation.
Clinical Outcomes
The use of the LHBT autograft for SCR is not a novel idea. Bush first described an open technique of biceps incorporation into the rotator cuff in 1975 (Bush 1975). He proposed that the hypertrophic and flattened LHBT could be redirected to serve as an alternate humeral head depressor in patients with irreparable MRCT. Nearly 20 years ago, Guven et al. also used an open technique, which consisted of enlarging the LHBT with split-thickness incisions parallel to the longitudinal axis prior to its tenodesis on the humeral head. Their series of 14 patients demonstrated a significant improvement in Constant scores, and satisfactory results were achieved in 85.7% of their patients.
More recently, Kim et al. used their arthroscopic “snake technique” to incorporate the LHBT in 53 shoulders with irreparable MRCT undergoing repair. At an average follow-up of 2.7 years, they discovered that 86.7% of patients had intact rotator cuff repairs. ASES scores also significantly increased from 60.9 to 82.7, and VAS pain scores improved from 4.1 to 1.0 (Fig. 6). Radiographic evaluation also demonstrated a significant increase in the acromiohumeral distance (AHD) by 2.7mm.
These findings are consistent with the study by Lee et al.. In their meta analysis comparing clinical and functional outcomes of patients undergoing SCR with different graft types, patients who underwent SCR with the LHBT had significant improvements in ASES scores (45.6 – 82.7), VAS-pain scores (5.2 – 1.4), forward flexion (139 - 164), and external rotation (42 – 51.4) at a mean follow-up of 27.2 months (Fig. 6). No significant differences were seen when compared to the other groups, which included HD allograft and tensor fascia lata (TFL) autograft.
Improved clinical outcomes were also observed in the cohort study by Kocaoglu, Firatli, and Ulku. Patients with MRCT underwent partial repair and SCR using either the LHBT (n = 14) or TFL autograft (n = 12). Pre-operative ASES scores in the partial repair SCR-LHBT group improved from 46.2 to 85.2, and VAS scores improved from 8.5 to 1.4 at a mean follow-up of 28 months after surgery. Forward flexion and external rotation also demonstrated significant improvement, with forward flexion increasing from 135° to 162.5° and external rotation increasing from 35° to 52.8° (Fig. 6). Radiographically, the AHD significantly increased after surgery in the SCR-LHBT from 7.0 +/- 1.5 mm to 10.2 +/- 2.5 mm (P = .02). At final follow-up there were no statistical differences in the ASES and VAS pain scores, range of motion, or AHD between the SCR-LHBT and SCR-TFL groups.
Improved healing has also been demonstrated when autologous LHBT is used for SCR in patients with MRCT. Barth et al. examined 82 patients with MRCTs who underwent repair by either a double-row technique (n = 28), transosseous-equivalent technique with absorbable patch reinforcement (n = 30), or an SCR with the LHBT autograft (n = 24). Ultrasound evaluation performed one year postoperatively showed that the supraspinatus and infraspinatus tendons remained intact in 91.7% and 100% of the patients in the SCR-LHBT group, respectively. This was statistically greater than the 60.7% and 74% seen in the double-row group and 56.7% and 76.5% seen in the transosseous synthetic patch group. Patients in the SCR-LHBT group had significantly improved strength compared to the other groups. ASES and VAS pain scores also significantly improved postoperatively (Fig. 6). However, there were no statistical differences seen in ASES, VAS, or Constant scores among the groups.
Llinás et al. compared clinical and structural outcomes in patients with MRCTs undergoing repair only (n = 50) or repair with a partial SCR using the autologous LHBT as a graft (n = 56). They found at 2-year follow-up, using ultrasonography, that the retear rate was significantly lower in the repair with partial SCR-LHBT group compared to the repair-only group (14% versus 46%; p < .01). ASES scores, VAS scores, forward flexion, and abduction were all significantly greater in the partial SCR-LHBT group as well (Fig. 6).
Discussion
The optimal treatment for young, active patients with irreparable MRCT without advanced glenohumeral degeneration remains unclear. The decision to perform partial repair, interpositional graft, SCR, subacromial balloon spacer, biological tuberoplasty, or RTSA all rely on patient age, range of motion, residual rotator cuff, and patient-specific requirements and expectations. There is no agreed-upon treatment algorithm nor comprehensive data to support one option over another (Kovacevic et al. 2020; Kucirek, Hung, and Wong 2021). We propose that the anterior cable procedure with the LHBT should be considered as an additional option within the MRCT algorithm.
Long disregarded or even discarded, the LHBT is a locally available and relatively easy-to-harvest autograft that provides several advantages. Unlike allografts, the LHBT is rich in live fibroblasts and viable tenocytes that can initiate stem cell differentiation into mature tenocytes (Colbath et al. 2022; Pietschmann et al. 2014; Tokish et al. 2022). The vascularity of the LHBT is also preserved when it is not tenotomized. This could promote greater graft incorporation and may help explain the higher rates of healing seen after rotator cuff surgery (Barth et al. 2020; Llinás et al. 2022; Fandridis and Zampeli 2020). The ACR contrasts Tokish’s novel description of a detached LHBT that is molded (smashed) into an augment patch (Tokish et al. 2022). The ACR with the LHBT procedure leaves the LHBT attached to the supraglenoid tubercle and translocated to the greater tuberosity. We will use the biceps as an ACR or “smashed graft” depending on the repairability, severity of the tear, and degree of tendinopathy.
From a biomechanical standpoint, improved outcomes may be seen in ACR with the LHBT for numerous reasons. The transposed biceps tendon may act as a bumper, or spacer, that limits the painful abutment of the greater tuberosity against the acromion—the bumper effect. This bumper effect continues to emerge as a method to reduce shoulder impingement in massive rotator cuff tears, whether balloon interposition, biologic tuberoplasty or bursal acromial resurfacing. Interestingly, we attribute the early pain relief in lower trapezius transfer to the bumper effect of the bulky Achilles.
The ACR may have a second potential mechanism of action acting as a static restraint against superior head migration, as borne out in the biomechanical studies reviewed above (Park et al. 2018; Berthold et al. 2021b; Krishnan et al. 2022). Although the methodology and testing mechanisms vary between studies, there appears to be a uniform restoration of joint mechanics and contact pressures. Graft thickness also plays an important role as demonstrated by Denard et al. where they found that SCR grafts thinner than 1 mm had significantly higher retear rates (60%) compared to grafts thicker than 3 mm (32%). The biceps is typically 5-6 mm (Denard et al. 2018) and may serve as a thicker autologous graft to reduce the risk of failure. Finally, we also propose that leaving the biceps intact distally during ACR may add a dynamic force to depress the humeral head, although this requires further biomechanical testing.
There is sound and consistent clinical support for the efficacy of ACR with the LHBT for patients with irreparable MRCT (Kim et al. 2021; Lee et al. 2021; Kocaoglu, Firatli, and Ulku 2020). It is unclear whether the biomechanical features of the ACR, the biological features of the autologous LHBT, or some combination of the two contribute to increased healing rates of surgically repaired rotator cuff tissue. Nonetheless, rotator cuff healing rates are enhanced by the biceps tendon.
Donor site morbidity, immune reactions, case complexity, and cost of procedures have also reinvigorated interest in the long head of the biceps as a source of autologous tissue (Veen, Stevens, and Diercks 2018). A recent retrospective review showed SCR to have a significant additional cost of $3,922 compared to lower trapezius transfer and RTSA (Marigi et al. 2022). The subacromial spacer, despite early promising results (Savarese and Romeo 2012; Familiari et al. 2021), may not be the best option either, given the cost and recent level I evidence demonstrating its inferiority to debridement alone (Metcalfe et al. 2022) and non-superiority when compared to partial repair (Verma et al. 2022). RTSA has also been a proposed treatment option in this difficult-to-treat population; however, data demonstrates reduced long-term survivorship, higher complication rates, and poor functional improvement in patients < 60 years old (Hartzler et al. 2015; Sevivas et al. 2017; Ernstbrunner et al. 2017). Finally, the LHBT has no additional donor site morbidity, eliminating the potential for infection and symptomatic harvest sites seen with fascia lata harvest for SCR (de Campos Azevedo, Ângelo, and Vinga 2018; Ângelo and de Campos Azevedo 2022).
It is important to recognize that ACR with the LHBT procedure, along with interposition grafts, SCR, tendon transfers, and even arthroplasty, are often salvage procedures in the young active patient. Most studies report scores that surpass MCIDs as well as 10-point increases in the ASES and PASS scores. However, expected outcomes are good, not typically excellent, with SSV or ASES scores in the '80s (Woodmass et al. 2019; Lim et al. 2018; de Campos Azevedo, Ângelo, and Vinga 2018; Hartzler et al. 2015; Sevivas et al. 2017). This is important to help set patient and surgeon expectations.
Identification of which patients will benefit from partial repair, interpositional graft, SCR, subacromial balloon spacers, biological tuberoplasty, or RTSA is currently a Jedi skill set. Nonetheless, identification of the patient’s goals and recognizing the limitations of each of these procedures is the first step in developing evidence-based algorithms.
Controversies
There are several concerns with using the LHBT as an autograft for patients with MRCTs. First, if the LHBT has been previously ruptured or tenodesed, then it can no longer be considered a graft option. This should be identified preoperatively, and preparations must be made to use alternative grafts. The LHBT may frequently appear diseased or degenerated, and the decision to use it is at the surgeon’s discretion. However, it is the author’s experience that even a structurally abnormal LHBT can allow for a successful ACR. Presently, studies are underway investigating the histologic and biomechanical properties of diseased tendons, which may help elucidate the appropriate treatment in the future.
Postoperative stiffness is an obvious concern when a tenodesis of the LHBT is performed and left intact. However, it is important to recognize that this subset of patients is very different from those with superior labrum anterior to posterior (SLAP) tears. Patients with irreparable MRCTs are typically not looking to return to competitive throwing; therefore, a slight loss of terminal external rotation may not be clinically relevant in this population. Furthermore, several series show no motion loss after ACR with the LHBT (Kim et al. 2021; Kocaoglu, Firatli, and Ulku 2020; Llinás et al. 2022).
The final controversy surrounds whether the LHBT should be left intact distally or cut and tenodesed below the repair. Theoretically, an intact LHBT may provide some superior shoulder stability and enhanced vascularity. The existing literature does not indicate whether the LHBT should be left intact or released; however, Adrian and Field suggested that the incidence of LHBT pain and bicipital groove pain is not higher in patients where the LHBT is left intact.
Conclusion
Anterior cable reconstruction using the autologous LHBT has shown promising results in patients with massive irreparable rotator cuff tears. This technique offers an alternative to traditional surgical approaches and has been shown to improve shoulder function, decrease pain, and increase patient satisfaction. Although there is still a need for further research to evaluate long-term outcomes and compare it with other treatment options, the available evidence suggests that this procedure is a viable option for patients with massive rotator cuff tears. As with any surgical intervention, proper patient selection and appropriate surgical technique are essential for successful outcomes. With further studies and refinement of techniques, the use of autologous LHBT for anterior cable reconstruction may become a standard treatment option for this challenging patient population.