Introduction
Proximal humerus fractures account for approximately 185,000 emergency department (ED) visits per year (Kim, Szabo, and Marder 2012). This fracture is more common in elderly patients, with the highest incidence in women 80-89 years of age (Court-Brown, Garg, and McQueen 2001). A 2015 study found that the incidence of proximal humerus fractures per 100,000 person-years was 82 (95 % CI 76 to 88). Proximal humerus fractures are three times more common in women than men. Many anatomical structures surround the proximal humerus, including nerves of the brachial plexus, the most significant of which being the axillary nerve; studies demonstrate up to a 67% likelihood of concomitant nerve injury (de Laat et al. 1994; Hems and Mahmood 2012; Visser et al. 2001; Warrender, Oppenheimer, and Abboud 2011) when proximal humerus fracture occurs. Numerous treatment methods are used to treat proximal humerus fractures, including both non-surgical and surgical options. Surgical methods including ORIF with locked plating and RTSA are preferred in many, but not all three and four-part fractures (Sanchez-Sotelo 2006).
RTSA has been demonstrated to improve complication rates and outcomes compared to open reduction and internal fixation (ORIF) in head to head studies (Greiwe et al. 2020). In 2015, a sample of 7714 patients with proximal humerus fractures drawn from the Nationwide Inpatient Sample database of 2011 found that 27.4% of patients were treated with rTSA (Schairer et al. 2015). By 2016, rTSA utilization in treatment of proximal humerus fractures had increased to 67.4% (Dillon et al. 2019). Despite the increase in utilization for most three and four-part fractures, treatment of fracture-dislocations of the proximal humerus with rTSA is challenging, as reduction of the tuberosity and maintenance of reduction are important for function and many of these injuries can present with concomitant neurologic injury. Preoperative brachial plexus, and more specifically, axillary nerve injury has been stated as a contraindication of rTSA due to hypothesized poor functional outcomes and increased rates of dislocation (Valenti et al. 2012).
While some proximal humerus fracture patients present to the emergency room (ER) with nerve damage, others seemingly develop more noticeable nerve damage between their admission to the ER and their preoperative evaluation. The question arises of whether rTSA is a suitable option for patients who present with a proximal humerus fracture with an associated or evolving nerve injury. However, studies are limited due to small patient populations and none have specifically addressed this particular question. The objective of this study is to evaluate the results of proximal humerus fracture patients who receive RTSA who have associated preoperative nerve injuries and compare results to patients without associated nerve injuries at a minimum of 2 years. We hypothesize that there will be no significant decrease in RTSA postoperative outcome scores in patients with associated preoperative nerve injuries compared to the control.
The objective of this study is to compare results of RTSA patients with preoperative nerve injuries to patients without nerve injuries at a minimum of 2 years.
Materials and Methods
This study was approved by the hospital’s local institutional review board. A retrospective case series of all proximal humerus fractures treated with rTSA from 2010 to 2018 by a single surgeon RGM was performed.
Patient preoperative data including patient age at the time of surgery, sex, time of injury to surgery, smoking status, body mass index (BMI), American Society of Anesthesiologists (ASA), and diagnosis were collected through a retrospective chart review. Patients were defined as having a nerve injury if they had an abnormal sensory exam, motor weakness such that 0/5 muscle strength was noted in the muscles tested at the time of surgery (inability to contract their deltoid, weakness with elbow flexion/extension, hand grip or finger abduction), or EMG documented evidence of nerve injury post-operatively.
Follow-up data including range of motion (ROM)—Forward Flexion (FF), Internal Rotation (IR), External Rotation (ER) were obtained at their last clinic visit. Range of motion was calculated in the office using manual techniques for range of motion measurement. Validated functional outcome measures including Simple Shoulder Test (SST), American Shoulder and Elbow Scores (ASES) were all collected. Patients who did not have functional outcome scores at a minimum of 2 years were contacted by a member of the research team to obtain these scores over the phone. Common complications of RTSA, including scapular notching, periprosthetic fracture, acromial fracture, and infection were also documented if reported.
Surgical Technique
Reverse total shoulder arthroplasty for fracture was performed in the beach chair position. A standard deltopectoral approach was utilized. Upon exposure of the humerus, the fracture lines were identified and exploited. A heavy strength #2 suture was applied to the tendon-bone junctions both anteriorly and posteriorly along the tuberosities. Typically, the fracture line was present just posterior to the bicipital groove and contained the subscapularis tendon. We then removed the head and exposed the glenoid by removing the labrum and cartilage. The glenosphere was placed in standard fashion and was a size 0 component with a 36 mm with an overall lateralization of 23.1 mm in all cases. The overall neck shaft angle was 152.5 degrees.
The stem size was chosen using a series of reamers and the stems were distally fitting stems placed in all cases without cement. The tuberosities were anatomically aligned in all cases and held in place with cerclage sutures. The height of the tuberosities were judged by reducing them anatomically to the shaft when the calcar was intact. In the setting where the calcar was not present, the tuberosities were placed 10 cm proximal to the pectoralis insertion and reduced to the superior aspect of the implant, such that the top of the tuberosities were in line with the top of the implant.
Participants
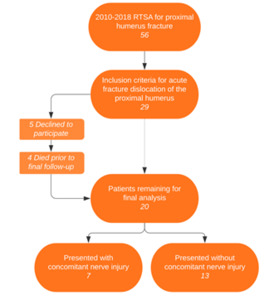
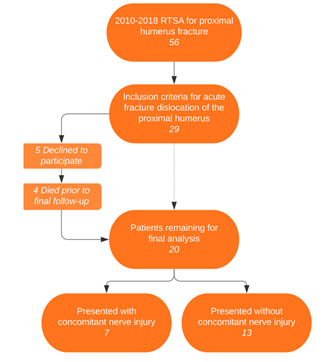
A total of 56 total RTSA’s for proximal humerus fractures were performed during the time period of this study. Of these 56, 29 patients treated with rTSA for fracture-dislocation were identified. All fracture-dislocation patients treated with rTSA were contacted. Five patients declined to participate in the study, 4 patients died prior to final follow-up, leaving 20 patients in the study group. Two patients were lost to follow-up leaving 18 of 20 patients (90%) with final follow up data. Seven (35%) patients presented with an overall brachial plexus, or more specifically, an axillary nerve injury associated with their fracture. Two of these seven had brachial plexus injury that were not identified as axillary nerve injuries, and the remaining 5 had axillary nerve injury. Three total patients got an EMG study done.
Statistical analysis
All statistical analysis was performed with GraphPad Prism (www.graphpad.com), and P < .05 was considered statistically significant. Univariate analysis of continuous variables was conducted with a Student t test.
Results
There were no differences between the two groups regarding age or BMI. Patients without nerve injury had a higher ASA compared to the patients in the nerve injury group 2.92 +/- 0.28 vs 2.43 +/- 0.53 (P=0.0130) but these values do not carry any clinical significance in regards to a practical difference in shoulder functionality. There was no difference in time to surgery after fracture between the 2 groups.
At the last final follow-up, there was no difference between the 2 groups regarding shoulder ROM, ASES or SST scores. Average follow-up was 5.83 years (range 2-8 years).
Coefficient of determination for patients’ outcome scores in relation to their age. Increased age correlated with lower ASES function and SST scores, but higher ASES pain scores.
Complications
No patients experienced any postoperative complications. However, three patients with a preoperative nerve injury were still reporting issues at the final follow up. One patient reported “discomfort and lack of motion”, but declined to get an EMG. This patient still had improved strength and ROM. Another patient had weakness in the upper extremity, however she also sustained a stroke affecting the side which is a confounding variable. The stroke occurred prior to surgery and was the reason this patient suffered a fracture-dislocation. The third patient still reported numbness and tingling from her elbow to her fingers. She had multiple workups including EMG demonstrating no evidence of mononeuropathy, polyneuropathy or radiculopathy despite an MRI demonstrating multiple cervical herniated discs. Overall only three total patients agreed to an EMG study. One of these three was a patient with a brachial plexus injury that was not axillary; the lesion was further identified on EMG as an infraclavicular brachial plexus lesion. Post-operative scores for this patient and the second patient with non-axillary brachial plexus injury were consistent with patients who had axillary nerve injury.
Discussion
Proximal humerus fracture patients with concomitant nerve injury did not show significant differences in ASES pain or function outcome scores after two years post-operation compared to proximal humerus fracture patients without concomitant nerve injury. In addition, there were no significant differences in forward flexion, external rotation, or internal rotation range of motion between the two groups. While three patients with concomitant nerve injury did report nerve-related complications after two year follow-up, these complications were also attributed to other confounding variables such as prior history of stroke and cervical herniated discs and radiculopathy.
The incidence of nerve injury in patients sustaining proximal humerus fractures varies significantly in the literature, ranging from as low as 6.2% to as high as 67% (de Laat et al. 1994; Hems and Mahmood 2012; Visser et al. 2001; Warrender, Oppenheimer, and Abboud 2011). Anterior fracture-dislocation demonstrates an increased incidence of neurologic injury versus other fractures of the proximal humerus. Of the 20 patients reported in our study, 7 (35%) presented with some form of concomitant nerve injury. Although older studies have reported that some patients did suffer from motor loss after surgery due to their nerve injury caused by the initial fracture (de Laat et al. 1994), a recent 2019 study showed that all patients with proximal humerus fractures with concomitant nerve injury showed full or partial neurological recovery (Gasbarro et al. 2019). This recent study is consistent with the data we gathered in our study, as all 7 of our patients exhibited either full or partial neurological recovery, or noted nerve damage due to pre-existing conditions or new injuries.
Traditionally, rTSA has been used over TSA in patients with significant rotator cuff injury or rotator cuff arthropathy, but recently indications have expanded to include severe arthritis with retroversion greater than 35 degrees and significant posterior subluxation, fractures of the proximal humerus and their sequelae, and even chronic instability. rTSA relies on the deltoid to assist in shoulder movement, hence reducing the need for a functional rotator cuff (Giles et al. 2015). Understandably, in patients with proximal humerus fractures with concomitant axillary nerve injury (Lopiz et al. 2016), rTSA was originally thought to be a contraindication due to the dependence this surgery has on the deltoid for successful range of motion and stability. Our study demonstrates that in a fracture-dislocation setting where nerve injury is common, rTSA may still be a good option for patients with concomitant nerve injury. Further large scale, multicenter studies on this topic would be beneficial to continue to demonstrate rTSAs effectiveness despite neurologic injury. These further studies could aim to show that the resultant stiffness and common return of overall brachial plexus, and more specifically, axillary nerve function assists in the prevention of instability and disability originally feared by prior authors (Kiet et al. 2015).
Similar to a 2016 study, patient age was found to be a negative predictor of rTSA outcomes in patients with proximal humerus fractures. While the study hypothesized that complication rates would decrease in the older population due to decreased demand, they found that higher complication rates were found in patients older than 80 years of age (n=26) compared to younger patients (n=16) (Lopiz et al. 2016). Similarly, we also found that an increase in patient age correlated with an overall lower total ASES and SST score although they had improved ASES pain scores, and lower ASES function scores.
A 2017 systematic review of rTSA performed on proximal humerus fractures found that the most common complication was dislocation (16.9%), followed by infection (6.7%), intraoperative fracture (3%), and neurological injury (2.6%) (Holton et al. 2017). However, a study published in 2013 found that of 49 rTSA patients with deltoid impairment, only two reported dislocation. The study went on to conclude that preoperative deltoid impairment was not an absolute contraindication to rTSA in proximal humerus fracture patients (Lädermann et al. 2013). This study did not however specify whether these patients had proximal humerus fractures; an emphasis was placed on deltoid impairment. Our study, although a smaller sample size of patients, is consistent with the results of this study, as we found that no patients had post-operational complications as a direct result of the pre-operative nerve injury.
A 2019 retrospective case series by Gregory Gasbarro, MD is the most similar study available for comparison of data to our data in this study. However, a few key differences in study design should be noted. The Gasbarro study used patients spanning from 2006 through 2018, while our study spanned from 2010 to 2018. In addition, the inclusion criteria for the Gasbarro study required participants to be over 65 years of age, while our study had no age limitations. The three participants in our study who were below the age of 65 (56, 63, and 64) averaged together had a higher SST and ASES fxn, while having a below average ASES pain compared to the participants 65 and older. The 2019 study tested QuickDASH, VAS, SSV, and forward elevation, while our study tested ASES function, ASES pain, SST, and RDM. Sixteen patients met the inclusion criteria for the Gasbarro study, and five presented with concomitant nerve injury. Twenty-nine patients met our study inclusion criteria, 18 patients presented follow-up data, and seven presented with concomitant nerve injuries. The 2019 study had an average follow-up of 3.1 years, with a range of one to five years, while our study had an average follow up of 5.83 years with a range of 2 to 8 years. Despite these differences, the two studies still managed to draw similar conclusions. The 2019 study states that “rTSA reliably restored overhead function, and overt nerve palsy did not lead to higher complication rates, including dislocation” (Gasbarro et al. 2019). Our study found no difference between patients with and without concomitant nerve injury regarding time to surgery, range of motion, ASES, or SST scores. Due to scarcity of data for rTSA’s performed on patients presenting with proximal humerus fractures with concomitant nerve injury, the addition of future similar cases could increase the confidence level of results from existing studies with greater statistical significance.
The opportunity for future studies is also vast in regards to RTSA for proximal humerus fractures with concomitant nerve injury. One such study that could be very beneficial to literature would be to compare the RTSA patients to non-operative treatment in terms of nerve recovery and functional outcome.
Limitations to this study include its small sample size. While neurologic injury is a rare complication of proximal humerus fractures and fracture-dislocation is rare as well, the small sample size still increases the possibility of a type II statistical error. In addition, a retrospective case series can be prone to recall bias, impacting recollection of post-operative complications. Chart notes were the primary method for identification of ongoing neurologic symptoms, but patients were asked whether they had any ongoing complications from the injury. Selection bias may have potentially been present due to the surgeon’s discretion on what patients would be eligible for the surgery, however no patients during this time period were selected for hemiarthroplasty or non-operative treatment after fracture-dislocation. Another limitation to the study is the lack of preoperative scores for comparison.
Conclusion
This study found no significant differences after two years post-operation in forward flexion, external rotation, internal rotation range of motion, or ASES pain outcome scores of patients with and without concomitant nerve injury. No patients experienced postoperative dislocations or any other postoperative complications that could be directly linked to the rTSA operation following nerve injury. Reverse total shoulder arthroplasty for fracture-dislocation in the acute setting may be a feasible option in patients with concomitant overall brachial plexus, and more specifically, axillary nerve injury. Further studies with higher numbers of qualifying patients are necessary to further understand the significance of these findings.



.jpeg)


.jpeg)