Introduction
Osteoporotic fragility fractures are associated with significant morbidity, mortality, and economic burden (Burge et al. 2006). As the population ages, the incidence of fragility fractures is predicted to increase, particularly among elderly individuals (Omsland and Magnus 2014) due to age-related decrease in bone mineral density, cognitive impairment, and impaired mobility (Egan et al. 2008). Having a fragility fracture substantially increases the incidence of a second hip fracture (Berry et al. 2007; Kanis et al. 2004; Colón-Emeric et al. 2003), and this effect cannot be explained by risk factors alone, as the incidence of repeat fractures are significantly higher than expected (Ryg et al. 2009). Due to the significant health and economic burden of fragility fractures, interventions to decrease the incidence of a repeat fracture should be implemented.
Many medical conditions and lifestyle factors increase osteoporotic fractures. The association between certain medications and primary fractures has been extensively studied. Medications related to an increased risk of fragility fractures include neurotropic drugs such as antidepressants and anxiolytics, sedatives and benzodiazepines, gastrointestinal drugs such as H2 antagonists and proton pump inhibitors, and endocrine drugs such as thyroid hormone replacement therapy (Mortensen et al. 2020). Another class of medications significantly associated with increased fracture risk are corticosteroids, which cause decreased bone mineral density (Vestergaard, Rejnmark, and Mosekilde 2005; Van Staa et al. 2000) by promoting apoptosis of osteoblasts and osteocytes while simultaneously stimulating osteoclast activity via upregulation of RANKL and downregulation of osteoprotegerin (Compston 2018). Inhaled and topical corticosteroids have minimal impact on increased fracture risk, but greater than 2.5 mg of systemic prednisolone equivalents per day is associated with an increased fracture risk (Vestergaard, Rejnmark, and Mosekilde 2005).
Because the association of corticosteroids and other medications with fragility fractures is well known, their use should be limited in high-risk patients unless necessary. Despite this, treatment with medications that increase fall risk is still widely used before and after osteoporotic fractures (Sjöberg et al. 2010), and there seems to be no change in their use after the initial fracture (Munson et al. 2016). While many studies have examined the association of medications and primary fragility fracture, few studies have explored the impact on repeat fractures. This study aims to explore trends in systemic corticosteroid use surrounding fragility fractures and their impact on repeat fragility fractures to improve treatment of at-risk patients.
Methods
Patients from the Research Action for Health Network (REACHnet) database were identified by having one of the fragility fracture codes seen in Table S1 from 2011-2015. If they had an associated trauma code in Table S1 within 7 days of fragility fracture, they were excluded. Repeat fractures were also excluded if a patient had a trauma code within 7 days of fragility fracture, leaving 11,373 patients. 2,598 patients were excluded for not having at least one year of medical history available before the initial fragility fracture. Another 5,770 patients were excluded for not having at least 2 years of follow-up time after the initial fracture or a subsequent fragility fracture within 2 years. 67 patients were removed for missing BMI, 24 were removed for being self-payers, 59 were removed for not having white or black race, 9 were removed for missing Hispanic ethnicity status, and 35 were removed for having a fracture in 2016, which is outside the time window. This left 2,643 patients in our cohort. Charleston Comorbidity Index (CCI) was calculated for each patient excluding age, since age was also used in corresponding regressions.
Categorical covariates are summarized by reporting count (%) and continuous covariates are summarized by reporting mean (sd). Categorical covariates are compared between re-fracture groups (no/yes) using a Fisher exact test, with continuous covariates compared using a two-sample t-test. Multivariable logistic regression is used to predict re-fracture within 1-2 years after initial fracture as a function of patient covariates to determine which factors are related to increased re-fracture risk, after adjustment.
The primary variables studied for an association with repeat fractures were timing of corticosteroid administration (before or initial fracture) and method of corticosteroid administration (oral or parenteral). Parenteral corticosteroids include intravenous and intramuscular injections. ICD codes for prescriptions indicative of intra-articular injections, including all prescriptions for triamcinolone, were excluded. The list of steroids and dosages are provided in Table S2. Demographic factors (race, ethnicity, and insurance type), risk factors (alcohol use, tobacco use, BMI, age, osteoporosis diagnosis, and CCI), and history of other drug use (bisphosphonates and PPI) were also studied to identify an association with corticosteroid use or repeat fracture. Finally, logistic regression was performed for patients who took each steroid type to determine if increased cumulative dosage was associated with an increased risk of re-fracture. All steroid types were converted into prednisone equivalents.
Results
Demographics
Table 1 displays the demographic characteristics of patients included in the study. Fragility fracture patients were mostly white, of non-Hispanic ethnicity, and had a diagnosis of osteoporosis (85.5%). They were also older and had a high adjusted CCI. Among the 2,643 patients with a fragility fracture, 1,125 (42.6%) of patients received corticosteroids within 1 year prior to fragility fracture or in the following two years. 676 (25.6% overall) fragility fracture patients received a parenteral corticosteroid, while 795 (30.1%) received an oral corticosteroid. Without adjusting for other covariates, patients on corticosteroids had a slightly higher but insignificant risk of repeat fracture (22.4% vs 19.2%, p=.052) compared to non-users. 465 (17.6%) patients were given corticosteroids before initial fracture and 968 (36.6%) of patients were given corticosteroids after their initial fragility fracture. Among the patients given corticosteroids after fracture, 588 (22.2% overall) were given parenteral corticosteroids and 635 (24.0%) were given oral corticosteroids.
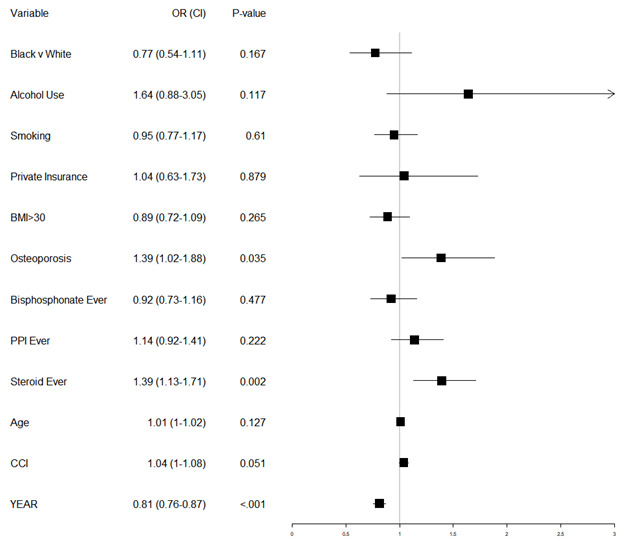
Patients who were given corticosteroids within this time were also significantly more likely to be African American (10.6% vs 7.6%, p-value=.011), have private insurance (7.2% vs 3.2%, p-value<.001), and be a smoker (34.9% vs 31.2%, p=.045). They were significantly more likely to have BMI >30 (49.7% vs 35.9%, p<.001), to use bisphosphonates (28.4% vs 19.4%, p<.001) or proton pump inhibitors (47.7% vs 26%, p<.001), and had a higher Charleston comorbidity index (3.43 vs 2.72, p<.001). Figure S1 displays results from a multivariable logistic regression predicting corticosteroid use. Significant predictors of corticosteroid use included BMI over 30, osteoporosis, bisphosphonate use, CCI, year, and the largest predictor, PPI use (adjusted odds ratio, aOR = 1.91, 95% CI = 1.61-2.28). Increased age was associated with a decreased odds that a patient received corticosteroids.
Corticosteroid use over time
Figure 1 displays corticosteroid use rates before initial fragility fracture (up to 1 year), after initial fragility fracture (up to 2 years), and two conditional corticosteroid use plots after initial fracture.
The percentage of patients with a fragility fracture increased over time, but this may be related more to the inclusion criteria. Patients with a fragility fracture had increased pre-fracture corticosteroid use over the years 2011-2015, from 3.3% to 30.1%. This upward trend is also seen for both oral and parenteral corticosteroids separately. Patients presenting with a fragility fracture were even more likely to receive a corticosteroid after fracture than before, with a rate increasing from 23.8% in 2011 to 50.1% in 2015. The two bottom plots examine the rates of corticosteroid use after initial fracture in two subsets of patients, those without corticosteroid use in the previous year and those with corticosteroid use. Patients who were not given corticosteroids before fracture were still given corticosteroids afterwards 23.4% of the time in 2011, which rose to 39.2% in 2015. Patients who were given corticosteroids prior to fragility fracture had even higher continued use over time, going from 33.3% in 2011 to 75.3% (in 215 qualifying patients) in 2015. The proportion of patients receiving parenteral corticosteroids in 2015 was also higher than those receiving oral corticosteroids (55.3% vs 49.3%).
There is a consistent pattern of prescribing more corticosteroids in these patients before initial fragility fracture, which may not be modifiable, and after fragility fracture, which should be modifiable behavior. Coupled with the conditional trends in the bottom of Figure 1, the odds of continued corticosteroid use after fracture are 4.51 times higher (95% CI = 3.63 - 5.62) for previous corticosteroid users. At the same time, the rate of re-fracture decreased over time overall, going from 25.5% in from 2011-2013 to 15.0% in 2014-2015.
Predictors of a second fragility fracture
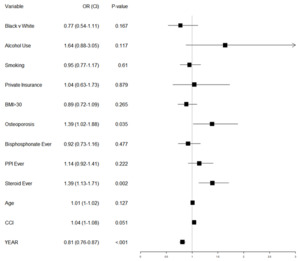
Table 2 displays the demographics of patients by refracture group, along with unadjusted p-values testing whether those demographics differ by group. We see no unadjusted significant differences by race, ethnicity, insurance status, smoking, alcohol use, or older age for refracture risk. We did see significant increases in refracture risk for those with osteoporosis (21.5% vs 14.9%, p=.004) and increased CCI (3.29 vs 2.95, p=.01). Dosage information for steroid users is also shown in this table but discussed later. Table 3 investigates whether there are different cofactors of corticosteroid use and refracture. In the corticosteroid cohort, no factors were significantly associated with refracture risk except CCI (3.75 average in the refracture group vs 3.33 in the no-refracture group, p=.045). There was an increased risk (but non-significant) in alcohol users (40.9%, p=.065, n=21). In the cohort of patients without corticosteroid use, there was a significant association between refracture and average age (79.73 vs 78.52, p=.037) and osteoporosis status (p=.005). Other than those listed above, there were no significant differences in demographics between refracture groups within the two corticosteroid groups. We attempted to adjust for these potential confounding variables via multivariable logistic regression to predict a second fragility fracture, with results shown in Figure 2. The p-value for testing whether this model fits better than an intercept only model is 2.89 E-11 (difference in deviance of 80 on 14 degrees of freedom) indicating that the model predicts secondary fractures much better than an intercept only model.
Corticosteroid use was associated with a significantly increased risk of a second fragility fracture (adjusted odds ratio, aOR = 1.39, 95% CI = 1.13-1.71). A diagnosis of osteoporosis showed similar adjusted odds of a second fragility fracture (aOR = 1.39, 95% CI = 1.02-1.88). Raising patient CCI had an increased, but not quite significant, odds of complication (aOR = 1.04, 95% CI = 1.00-1.08, p-value = .051). A later year of initial fragility fracture was associated with a decreased risk of re-fracture (aOR = .81, 95% CI = .76-.87). Neither bisphosphonate use nor PPI use over the time period were associated with a significant change in risk of repeat fracture.
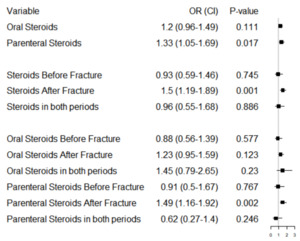
Figure 3 displays the adjusted odds ratios and associated 95% confidence intervals for the steroid effect broken down by administration method (oral vs parenteral), timing (steroids given before initial fragility fracture, after, and their interaction), and the administration method and timing combination (i.e. oral steroids before, after, interaction; parenteral steroids before, after, interaction). When considering administration method only (before or after initial fracture), parenteral corticosteroids were associated with significantly increased odds of re-fracture (aOR = 1.33. 95% CI = 1.05-1.69) but oral corticosteroids were not, despite showing slightly increased adjusted odds of re-fracture (aOR = 1.20, 95% CI=.96-1.49, p-value=.111). In terms of timing, only corticosteroids administered after initial fracture were associated with an increased odds of re-fracture (aOR = 1.50, 95% CI = 1.19-1.89). There was no apparent interaction effect for patients receiving corticosteroids in both time periods. When considering timing and administration method together, only parenteral corticosteroid use after fracture was associated with an increased risk of re-fracture (aOR = 1.49, 95% CI 1.16-1.92). Oral corticosteroid use after initial fragility fracture had slightly, but not significantly, increased odds of re-fracture (aOR = 1.22, p-value = .123). There was no significant interaction effect in corticosteroid timing for either type.
Necessary corticosteroid use
Corticosteroids are a widely used medication, but there are only some medical conditions in which corticosteroids are the standard of treatment. A list of conditions where corticosteroid use was indicated, and therefore the benefit outweighed the potential increased fracture risk, is given in supplemental Table S3. Our results showed that only 44.4% of patients with parenteral corticosteroid use and 53.1% of patients with oral corticosteroid use had a diagnosis code within 7 days of corticosteroid prescription that required corticosteroid use.
Corticosteroid Dosage
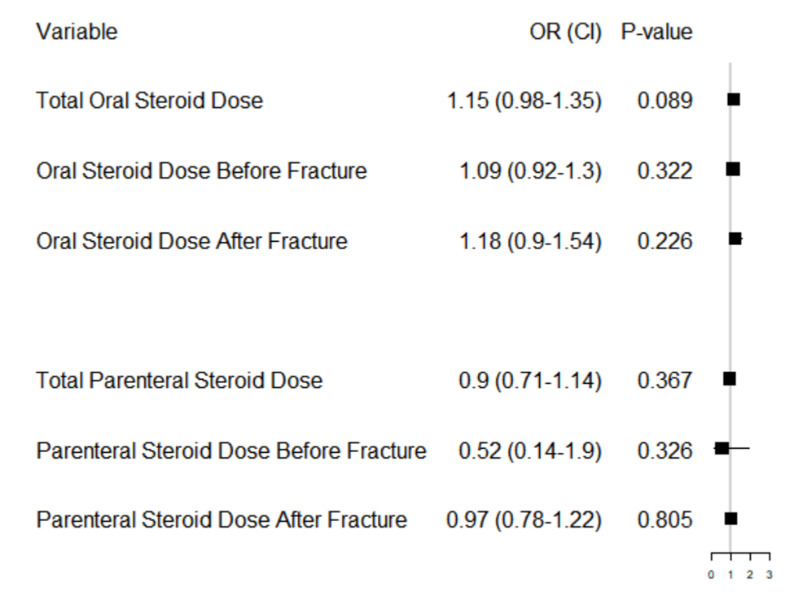
Next, dosage of steroids was analyzed to see its effects on refracture risk among the set of patients who did receive steroids. Dosages were standardized to prednisone equivalents. Table 2 shows the mean dosage of prednisone equivalents for parenteral and oral steroids before fracture, after fracture, and in total. An increased cumulative dosage of prednisone equivalents administered parenterally before fracture was significantly associated with a decreased risk of repeat fracture (P=0.009). Dosage did not have a significant effect for any of the other categories.
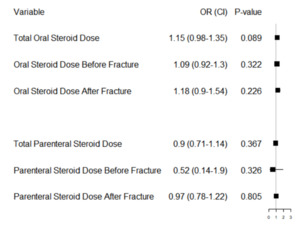
Figure 4 displays the dose-effects of 6 different logistic regressions. These were (1) an adjusted model with total oral steroid dosage over the study period, (2) an adjusted model with oral steroid dosage before first fracture, (3) an adjusted model with oral steroid dosage after first fracture, (4) an adjusted model with total parenteral steroid dosage over the study period, (5) an adjusted model with parenteral steroid dosage before first fracture, (6) an adjusted model with parenteral steroid dosage after first fracture. Only patients that took each steroid type within the time frames considered were included in these analyses (i.e. decreased sample sizes for each analysis). We did this to use the most patient data when studying each dosage effect across different time periods. In this analysis, cumulative oral steroid dose equivalents trended towards an increased risk of repeat fracture but did not reach significance (p=0.089). This analysis also revealed that increased parenteral administration before fracture did not influence repeat fracture risk, contrary to the initial analysis.
Discussion
Osteoporotic fractures are associated with high morbidity, mortality, and economic burden, so decreasing their incidence is crucial for patient health and well-being. Many patients with osteoporosis are older and more likely to be taking medications for other medical conditions, including medications that may increase risk for fragility fractures, including corticosteroids. In our patient population consisting of patients with a fragility fracture, 42.6% of them received a corticosteroid within one year of the fracture, indicating that it is a widely used medication, even in the osteoporosis patient population. Further, the percentage of patients in our population taking corticosteroids increased from 2011 to 2015, indicating that they are becoming even more common. Due to the widespread use of corticosteroids, it is important to know if the risk of a repeat fracture outweighs the benefit of the anti-inflammatory effects.
We found that oral corticosteroid use was not significantly associated with a repeat fracture, which was contrary to our hypothesis. This finding is consistent with Munson et al (2018). They did find that stopping oral corticosteroids after the initial fracture decreased the risk of a repeat fracture, but it was only significant in non-bisphosphonate users. Their hypothesis for the lack of significant increased repeat fracture risk is that corticosteroids’ effects on bone mineral density take significant time, making the study window possibly too short to see the effects. Because of this, they argue that an increased repeat fracture risk may not need to alter treatment plans of oral corticosteroids in the short term.
However, Munson et al did not study parenteral corticosteroid use. Unlike oral corticosteroids, our study found that parenteral corticosteroids significantly increased repeat fracture risk, especially if given after the initial fracture. A possible explanation is that patients who receive parenteral corticosteroids are in the hospital and are therefore more likely sicker than patients who receive oral corticosteroids, which may make them prone to repeat fractures.
We hypothesized that an increased dosage of corticosteroids may increase the risk of repeat fractures because it has been shown that a higher dose of corticosteroids causes a linear increase in side effects (Huscher et al. 2008). However, after adjustment, our results did not reveal a significant effect of cumulative steroid dosage on repeat fracture risk. This finding is consistent with a previous study, which found that cumulative dose did not significantly alter primary fracture risk after adjusting for patient variables that may be confounders (van Staa et al. 2000). The lack of significant findings may indicate that a dosage effect on repeat fractures may not be present, but steroid use (including timing/method of administration) in general does.
Corticosteroids are a widely used medication, and our study showed that a significant proportion of patients received corticosteroids for another medical condition. However, only certain medical conditions indicate definite corticosteroid use [Table S3] (Hodgens and Sharman 2021). Only about half of our patient population had a diagnosis within 7 days of the corticosteroid administration that required its use. Because of the high use of corticosteroids and the known physiologic mechanism of increased fracture risk, it is critical to know if these medications should be avoided in high-risk patients. Further evaluation is needed to see if there is a definite effect of systemic corticosteroids on repeat fracture risk, and if there is a dosage threshold that may be safe for patients. Physicians should take caution with the use of unnecessary parenteral corticosteroids, especially if they had not been taking them before the initial fragility fracture, in order to prevent a repeat fracture.
There are several limitations to this study. One limitation is relying on electronic medical record coding for the data. We evaluated “necessary” corticosteroid use by having a diagnosis code within 7 days of the prescription, but it is possible that they were appropriately prescribed based on the risk/benefit analysis in the specific clinical scenario. This likely would increase the number of patients that received “necessary” corticosteroids; however, it still would not reach 100%, indicating that corticosteroids still may be overprescribed in this patient population. Additionally, because of limitations in ICD coding specificity, our study did not consider re-fractures within a 1-year window after initial fracture. ICD-9 coding does not differentiate between initial or subsequent encounters for a fracture. Therefore, the same fracture code could repeat at subsequent clinic visits and would falsely appear as repeat fractures. To be on the safe side, repeat fractures were not considered until after this one-year time period. As a result, it is possible repeat fractures that occurred were not included in the study. The goal of the study was to determine effects in medication alterations between primary and secondary fractures. Less than a one-year window between fractures would be too short of a time period to appreciate effects of drug adjustments, and thus this limitation does not impact the overall study goals. Another limitation is the time frame of the study, where we could only allow for 2 years of follow-up after the initial fracture based on the available data. With a longer time, more repeat fractures and a possible increased association with oral corticosteroids may have been seen.
In conclusion, fragility fractures are associated with significant morbidity and mortality, so decreasing their incidence is critical for patient outcomes. Our study found that parenteral corticosteroids, but not oral corticosteroids, especially when given after the initial fragility fracture, increased the risk of a subsequent fracture. Our data suggested that given steroid use, the dosage of steroids may not play a significant role in complication. This means that the decision whether to administer steroids at all may be more important than the amount, given necessity to use steroids. In the future, studies can investigate if higher doses or longer time of administration directly increases the fracture risk. Physicians may need to take caution in overprescribing parenteral corticosteroids in osteoporotic patients at high risk for repeat fragility fractures.
Acknowledgements
The research reported in this work was conducted in partnership with Research Action for Health Network (REACHnet), funded by the Patient Centered Outcomes Research Institute® (PCORI Award RI-CRN-2020-008). REACHnet is a partner network in PCORnet®, the National Patient-Centered Clinical Research Network, which was developed with funding from PCORI®. The content of this work is solely the responsibility of the author(s) and does not necessarily represent the views of other organizations participating in, collaborating with, or funding REACHnet or PCORnet®, or of PCORI®. The authors acknowledge the participation of REACHnet partner health systems in this project.
Author Bios
Connect with Justin David on LinkedIn
Conflicts of Interest Statement for Justin David
Gregory Benes is currently a third year medical student at Louisiana State University Health Sciences Center in New Orleans, LA. He is interested in pursuing a career in orthopedics.
Connect with Gregory Benes on LinkedIn
Conflicts of Interest Statement for Gregory Benes
Dr. Dasa currently serves as vice chair for academic affairs for the department of orthopedics, Irvin Cahen endowed chair for research, chair of the LSU clinical practice leadership group. He is the co-founder and chief medical officer for an innovative start up, SIGHT Medical and helped co found a novel medical education platform called DOC SOCIAL.
His research interests cover all areas of adult orthopaedics focusing on joint replacement, knee arthritis, health disparities and outcomes research. He is one of the few surgeons in the world performing outpatient opioid free total knee replacements allowing patients to recover in the shortest time possible.
Visit the Open Payments Data Page for Dr. Dasa
Conflicts of Interest Statement for Dr. Dasa
Dr. Krause is an orthopedic trauma surgeon with LSU in New Orleans Louisiana.
Visit the Open Payments Data Page for Dr. Krause
Conflicts of Interest Statement for Dr. Krause
Dr. Lauren Leslie is part of the Ochsner Sports Medicine Institute as a neuromusculoskeletal and sports medicine specialist. She completed her neuromusculoskeletal medicine residency at Michigan State University, where she served as chief resident and received the American Academy of Osteopathy award as resident of the year. Dr. Leslie completed her sports medicine fellowship at Edward Via Virginia College of Osteopathic Medicine/Virginia Tech, where she served as an associate team physician for Virginia Tech and Radford University athletics. She now serves as the team physician for Loyola University New Orleans as well as many local high schools. Dr. Leslie’s primary specialty is neuromusculoskeletal medicine. She has a specific interest in osteopathic manipulative medicine including osteopathy in the cranial field, movement biomechanics, and concussion management and research. She has training in both diagnostic and interventional ultrasound techniques, including orthobiologic injections.
Visit Dr. Leslie’s Website
Visit the Open Payments Data Page for Dr. Leslie
Conflicts of Interest Statement for Dr. Leslie
Dr. Deryk Jones is head of the Ochsner Sports Medicine Institute (OSMI), a program that recently joined with Dr. James Andrews to form the Ochsner Andrews Sports Medicine Institute. He double majored in biology and philosophy at Emory University and played collegiate soccer there as well. He received his M.D. degree from Stanford University where his interest and focus on articular cartilage research began. Orthopaedic training was at the Harvard Combined Program completing a prestigious Chiefship at the Massachusetts General Hospital in 1997. He then performed a fellowship in Shoulder Surgery and Sports Medicine at the University of Pittsburgh in 1998 and on completion joined the staff at Tulane University Medical School; he currently maintains appointments in Orthopaedic Surgery as a Clinical Assistant Professor at Tulane University and a Full Professor at the University of Queensland, Australia. He is a fellow of the American Academy of Orthopaedic Surgeons and the American Orthopaedic Association. He is board certified in orthopaedic surgery and subspecialty certified in sports medicine through 2030.
In 2004, he created OSMI and runs a program with over 160 athletic trainers, numerous operative and non-operative clinicians, physical therapist, and performance trainers. The program has locations throughout New Orleans and the state of Louisiana as well as the Mississippi gulf coast. He directs an operative fellowship training three board certified orthopaedist and one research fellow in sport medicine per year. OSMI also has residency programs in sports medicine assistantship and sports medicine physical therapy as well. He directs research focused on outcomes in shoulder, elbow, knee, hip and foot and ankle pathologic conditions evaluating operative and non-operative options and has published numerous articles on these subjects. He specializes in treatment techniques utilizing biologic reconstructive surgery and has been on the leading edge of innovations in regenerative medicine. His specific focus is on the treatment and regeneration of articular cartilage injuries.
Visit Dr. Jones’s Website
Connect with Dr. Jones on LinkedIn
Visit the Open Payments Data Page for Dr. Jones
Conflicts of Interest Statement for Dr. Jones
Dr. Chapple’s primary role as Director of Biostatistics is planning cancer clinical trials in mice, humans, and cell lines – while also executing statistical analyses. Dr. Chapple is an expert in adaptive clinical trial designs, particularly Bayesian methods. His 2019 paper “A hybrid Phase I-II/III clinical trial design allowing for dose re-optimization in Phase III” proposed a new framework for seamless drug development through the three stages of clinical trial design. This paper was recognized globally by the statistical community when it received the award for “Best Paper” from Biometrics, a top tier statistical journal. Dr. Chapple has received worldwide recognition for his contributions to adaptive clinical trial design methodology and he hopes this expertise can propel his institution towards innovative cancer therapies, rapid advances in treatment of chronic diseases, and the long-term goal of personalized/precision medicine.
Dr. Chapple has produced many new trial designs and methods, which are accompanied with user-friendly R packages. Dr. Chapple trained under Dr. Peter Thall at MD Anderson, who is a pioneer in Bayesian adaptive trials. He later refined these skills working with cancer researchers from other institutions. Dr. Chapple’s adaptive trial designs were used to dose-find in pediatric brain tumors in a trial conducted at Harvard and the Dana Farber Cancer Center.
Dr. Chapple has designed hundreds of studies and has supported many research departments at LSUHSC including: Orthopedics, OBGYN, Hematology Oncology, Dermatology, Oral Surgery, Endodontics, Cancer, Virology, Cellular Biology, Inflammation, and treatment disparities research. As a joint professor in Orthopedics, Dr. Chapple works on large scale electronic records databases which requires processing longitudinal information from patients on hospital encounters, diagnoses, procedures, prescriptions, and lab values.
Visit Dr. Chapple’s Website
Connect with Dr. Chapple on LinkedIn
Conflicts of Interest Statement for Dr. Chapple



_before_initial_fragility_fracture__(top_right)_aft.png)



_before_initial_fragility_fracture__(top_right)_aft.png)