Background
Open shoulder arthroplasty surgeries, including reverse total shoulder arthroplasties (rTSA), have an increased risk of bleeding, hematoma formation, and subsequent risk of a periprosthetic joint infection, which surgeons must seek to avoid at all costs. The risk of developing a healthcare associated infection (HAI) in patients undergoing surgery has been documented at 2.8 per 100 surgeries, with over 500,000 occurring each year in the USA. This can lead to prolonged hospitalization, higher costs, and higher rates of morbidity and mortality (Jarvis 1996; Coello et al. 2005; Whitehouse et al. 2002). In the shoulder, the most isolated pathogens are Cutibacterium acnes (C. acnes), Staphylococcus epidermidis, and Staphylococcus aureus (Atesok et al. 2017).
Appropriate sterilization techniques in the operating room are often overlooked, however they are vitally important for decreasing the risk of an HAI. The use of chlorhexidine gluconate with isopropyl alcohol (ChloraPrepTM) has been shown to decrease the bioburden of resident bacteria, including C. acnes, compared to other skin preparation solutions (Savage et al. 2012; Saltzman et al. 2009; Morrison et al. 2016).
Hemostasis in the setting of open orthopaedic procedures is an important variable that must be considered when devising a surgical plan. Numerous options exist for controlling intra-operative and post-operative bleeding related to surgery, and surgeons must be familiar with the options they have available to them to help assist with hemostasis.
Case Presentation
The patient is a 60-year-old right hand dominant male who presented with worsening chronic right shoulder pain. He reported eight out of ten pain, and disruption of his quality of life, including bathing, dressing, and sleeping. Under the care of his previous surgeon, he had undergone a right shoulder arthroscopic double row rotator cuff repair in 2013 which had failed. In 2018, he underwent a revision open right double row rotator cuff repair via a “Saber” incision approach, which also failed. For his chronic right shoulder pain, he has been treated with multiple rounds of physical therapy, oral medications, and five right subacromial corticosteroid injections. These modalities had failed to provide meaningful pain relief. His last injection was nine months ago. Pertinent past medical history included: rheumatoid arthritis, psoriatic arthritis, hypertension, obstructive sleep apnea, and type two diabetes mellitus.
Current imaging of his right shoulder displayed a recurrent right shoulder rotator cuff tear with arthropathy, superior humeral head migration, and signs of moderate degenerative glenohumeral joint disease. After consultation on the pros and cons of surgical intervention, the patient was consented for a right rTSA.
Infection Considerations
Pre-Operative Period
Prosthetic joint infection is a concern in this patient, given his multiple arthroscopic and open shoulder procedures. A surgical plan to decrease the risk of infection pre-operatively, intra-operatively, and post-operatively was instituted.
Given his previous surgeries, which included the use of multiple braided sutures, lab work (ESR, CRP, CBC) was obtained to rule out an active infection. Since these labs came back within normal limits, the decision was made not to aspirate the shoulder preoperatively. Methicillin resistant staphylococcus aureus (MRSA) and methicillin sensitive staphylococcus aureus (MSSA) nasal swabs were performed two weeks prior to the procedure. Treatment with five days of Mupirocin ointment was instituted intra-nasally twice per day due to a positive MRSA swab. Beginning 72 hours prior to surgery, chlorhexidine wipes were used to the planned surgical area by the patient at home. Additionally, dental clearance was obtained, and nutritional optimization was undertaken through tight glycemic control and nutritional shakes beginning two weeks prior to the planned surgery.
Skin Preparation
Our sterilization techniques included wearing clean scrub attire, decreasing operating room traffic, and changing to fresh sterile gloves frequently. Clipping of the body hair at the surgical site was undertaken prior to the skin preparation. In addition, we utilized chlorhexidine gluconate with isopropyl alcohol (ChloraPrepTM) to assist with decreased the risk of a C. acnes infection.
Bleeding Control
In the case presented, the patient had a history of chronic nonsteroidal anti-inflammatories (NSAIDs) for controlling his right shoulder pain as well as a daily 81mg aspirin.
Tranexamic acid (TXA) is an anti-fibrinolytic agent that can be administered either orally or intravenously. Its mechanism is at the level of the common pathway of the coagulation cascade. It is a synthetic lysine analogue which inhibits fibrinolysis promoting clot stability and may help reduce inflammation blocking the breakdown of fibrin (Reed and Woolley 2015).
AristaTM is a plant based, absorbable, topical hemostatic power derived from plant starch. It contains microporous polysaccharide hemospheres (MPH), a patented blood clotting technology, which allows for dehydration of blood upon contact and the creation of a bleeding barrier. AristaTM is delivered via a sterile bellow as a fine, dry, sterilized product that is compatible with human tissue. It is recognized by the body as native and is typically absorbed within 48 hours (Antisdel, West-Denning, and Sindwani 2009). Several randomized controlled trials have demonstrated the efficacy of MPH in a range of surgical settings (Antisdel, West-Denning, and Sindwani 2009; Antisdel 2008; Murat et al. 2004; Egeli et al. 2012).
The lead author’s preferred technique is to have one gram of TXA administered intravenously prior to incision and a second dose of one gram of TXA given at the time of implant placement. AristaTM is used throughout the case to manage bleeding from muscle oozing, mild to moderate bleeding during soft tissue resection, and during closure. The synergistic benefits of TXA and AristaTM, in his opinion, leads to less blood loss, a better visual field, and less post-operative hematoma and seroma as a potential source for a surgical site infection (SSI).
Surgical Details
The patient received a pre-operative interscalene brachial plexus nerve block by the anesthesia team under ultrasound guidance with liposomal bupivacaine (ExparelTM) to minimize intra-operative and post-operative pain. The patient received one gram of vancomycin and one gram of ceftriaxone pre-operatively. This has been shown to be an effective pre-operative prophylactic treatment regimen for minimizing C. acnes infections in patients undergoing shoulder arthroplasty (Portillo et al. 2013). The patient was also given one gram of TXA prior to incision.
A standard deltopectoral approach was used and the cephalic vein was taken laterally. AristaTM hemostatic powder was utilized when bleeding was encountered while the biceps was tenodesed to the upper border of the pectoralis major insertion. The powder was applied in a broad spectrum (Picture 1) at the site of bleeding in the inferior aspect of the wound and a lap sponge was applied with packing pressure.
Surgical exposure and release of the subscapularis tendon was performed while the sponge was maintained for 90 seconds. Next, the osteophytes surrounding the humeral head were resected and a free hand humeral head cut was made. The previous rotator cuff sutures and anchors were encountered at this point and removed to decrease to risk of infection by removing any previous niduses for infection. Since no frank pus or signs of infection were present during the case, the decision was made not to obtain intraoperative cultures at the time of surgery.
Attention was next turned to the glenoid. Oozing bleeding was encountered during the resection of the inferior labrum, as well as during capsular dissection around the subscapularis. Hemostatic powder was utilized around the glenoid and deep to the subscapularis to minimize oozing and maintain visualization (Picture 2). The inferior retractor was removed, and again hemostatic powder was placed around the inferior capsule. A lap sponge was used to apply packing pressure and the inferior retractor was again placed at the inferior aspect of the glenoid. This technique allows for optimal visualization while working around the axillary pouch and inferior glenoid without surgical delay.
After completing the glenoid preparation, application of a sterile povidone-iodine (PVP-I) wound irrigation solution (BD SurgiphorTM) was placed into the surgical wound while the glenoid implants were being opened (Picture 3). The solution is a proprietary, terminally sterile mixture containing 0.5% PVP-I formulation with 0.9% saline, potassium iodide, phosphate buffer, and vitamin E tocopherol polyethylene glycol succinate (TPGS). Prior to glenosphere implant placement, the solution was evacuated from the wound with suction and the area was thoroughly irrigated with 0.9% saline rinse (BD SurgiRinseTM).
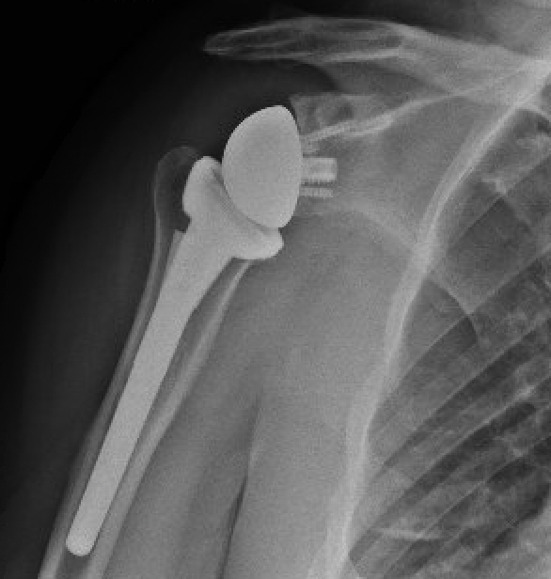
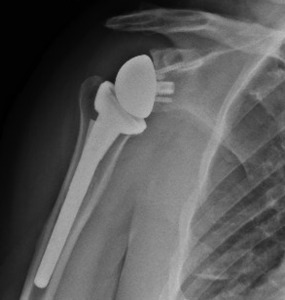
With the glenoid completed, attention was returned to the humeral head preparation. After identifying optimal implant sizing, the trial components were removed, and the canal was lavaged with double antibiotic pulse lavage. The SurgiphorTM and SurgiRinseTM steps were again repeated, and vancomycin powder was then placed down the humeral canal prior to final implant placement. It should be noted that surgical gloves were changed by the entire sterile field team prior to implant placement throughout the case. A final range of motion and stability check was performed, and the deep wound was irrigated with the pulse lavage. Prior to closure of the deltopectoral interval, hemostatic powder was placed into the deep wound and a second dose of one gram of TXA was given (Picture 4). After deltopectoral closure, the superficial wound was irrigated again using the SurgiphorTM and SurgiRinseTM combination. The remaining hemostatic powder was then placed in the subcutaneous fat layer to decrease postoperative seroma and adhesion formation. The wound was closed in a layered manner and a running Monocryl suture on the skin, followed by a watertight sealant glue. The patient was discharged to home on a 10-day course of oral doxycycline, 100mg BID. Radiographs were taken at the first postoperative visit two weeks after the procedure (Picture 5).
Discussion
Infection complications and blood loss are devastating complications in orthopedic surgery. Several touchpoints during the preparation, execution, and post-procedure care process can be affected by the surgical team to help mitigate the risk of infection and bleeding complications. Our case discusses the spectrum of care measures in our safety plan, with an emphasis on the surgical procedure.
Pre-operative use of intravenous vancomycin and ceftriaxone has been shown in the shoulder arthroplasty literature to help reduce the risk of C. acnes and other common shoulder infections. Furthermore, the use of vancomycin powder does the humeral canal has been shown to be a cost-effective way to minimize the development of SSI while decreasing the risk of developing resistant to cephalosporins (Hatch et al. 2017). The addition of a ten-day course of oral doxycycline further adds protection within this space.
Strategic intra-operative utilization of SurgiphorTM, a terminally sterile 0.5% povidone-iodine wound irrigation solution during the placement of implants and during wound closure offers a ready to use solution. Efficacy of dilute povidone-iodine solutions during spine and arthroplasty surgeries have shown a 3.4% reduction in SSI rate and 84.5% reduction in periprosthetic joint infections (PJI), respectively (Cheng et al. 2005; Brown et al. 2012). However, “back-table” compounding of such solutions can be time consuming and inaccurate. The ready to use, pre-mixed solution offers a safe and effective alternative to this by allowing for consistent concentrations of povidone-iodine. Furthermore, the PVP-I preservative in the solution has a 99.9% reduction rate in MRSA and Escherichia coli (E. coli) at 15 seconds using a time to kill measurement and exhibits minimal cytotoxicity when compared to Irrisept and Bactisure at 24 hours (“SURGIPHOR Solution Preservative Effectiveness Report” 2019).
The combination of TXA and a topical hemostatic powder (AristaTM) may provide a synergy for the reduction of intra-operative blood loss while maintain a clear surgical field. Additionally, it aids in the reduction of post-operative seroma and hematoma. The effectiveness of this, in the lead author’s opinion, helps decrease lost surgical time, eliminates the need for a post-operative drain, and protects the skin against drainage and potential wound complications. Post-operative hematoma or seroma development within the “dead space” from rTSA has been documented within the literature to potentially lead to fluid collection after a shoulder arthroplasty. This has been associated with higher rates of positive cultures and increased risk for periprosthetic joint infection. These inopportune outcomes often lead to poor clinical outcomes, increased morbidity (including the need for reoperation with an incision and drainage or possibly a two-stage antibiotic coated cement spacer with long term IV antibiotics) and mortality, and increased overall hospital costs (Cheung, Sperling, and Cofield 2008). In particular, previous research has shown that the average total cost of treating a periprosthetic joint infection after a shoulder arthroplasty is on average approximately $50,23019; therefore, we believe that utilizing prophylactic strategies to prevent a periprosthetic joint infection intra-operatively with the use of these novel products is of the utmost importance.
Conclusion
Successful outcomes in shoulder arthroplasty can be defined on many levels. Just as important as surgical technique is the effective institution of infection and blood loss prevents methods. The ability to utilize a multifactorial approach to provide patients with the best possible chance of a positive surgical outcome is incumbent upon the surgeon. Optimal outcomes in high-risk patients can be achieved through appropriate pre-operative patient optimization, intra-operative infection prevention, and understanding the role or proactive hemostasis.
Abbreviations
rTSA = reverse total shoulder arthroplasty; HAI = healthcare associated infection; C. acnes = Cutibacterium acnes; ESR = Erythrocyte Sedimentation Rate; CRP = C-Reactive Protein; CBC = Complete Blood Count; MRSA = Methicillin resistant staphylococcus aureus; MSSA = methicillin sensitive staphylococcus aureus; BID = twice per day; NSAIDs = nonsteroidal anti-inflammatories; TXA = Tranexamic acid; MPH = microporous polysaccharide hemospheres; SSI = surgical site infection; PVP-I = povidone-iodine; TPGS = tocopherol polyethylene glycol succinate; E. coli = Escherichia coli;
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Availability of Data and Materials
Not applicable.
Competing Interests
S.M. is a paid consultant of Becton, Dickinson and Company (BD), however he nor any of the authors were compensated for the creation of this manuscript.
Funding
No specific finding was received to complete this study.
Author’s Contributions
P.S. assisted with the surgical case and conception of creating this case report. He wrote the initial manuscript in conjunction with L.F. L.F., B.F., and W.B. assisted P.S with the writing and revising the manuscript. S.M. was the lead surgeon on the case, helped conceptualize creating this case report, oversaw the manuscript writing, and approved of the final version of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.




_wound_irrigation_system_was_placed_into_.jpeg)





_wound_irrigation_system_was_placed_into_.jpeg)