Introduction
Subscapularis management during shoulder arthroplasty continues to be an area of controversy (Collin et al. 2022). While catastrophic failure of the subscapularis tendon requiring revision surgery ranges between 5-6% of all cases (Boileau et al. 2006), loss of clinically detectable subscapularis function is present in 67.5% of patients following shoulder arthroplasty (Miller et al. 2003). Other complications include loss of motion, hematoma, weakness, anterosuperior escape, and instability. Revisions to reverse total shoulder arthroplasty are costly, estimated at $22,000 per revision procedure, and lead to worse outcomes compared to primary arthroplasty (Saltzman et al. 2014).
Many clinical studies have shown patients with subscapularis re-tears following primary arthroplasty surgery show lower satisfaction rates, lower shoulder function scores, and decreased internal rotation strength and range of motion. The healing of the subscapularis tendon is therefore critical to optimal shoulder arthroplasty results. Despite this, while shoulder arthroplasty has evolved to meet the early challenges of glenoid loosening and bony deformity, little progress has been made with understanding subscapularis healing and failure rates (Schrock et al. 2017). Ultrasound studies show that about 50% of patients undergoing shoulder arthroplasty experience a subscapularis re-tear that often is difficult to detect by physical exam or routine post-operative imaging (Miller et al. 2003). With the rapid increase in the number of annual shoulder arthroplasty procedures (100,000/year with 7% increase projected year-over-year) (Shoulder Replacement Market, n.d.), there remains a tremendous opportunity for surgeons to improve outcomes and decrease failure rates. The ability to improve subscapularis healing with biologic augmentation may represent a significant advancement in the treatment of the subscapularis tendon during shoulder arthroplasty.
As tendons are composed of collagen fibers that are uniaxially aligned and wavy, replicating the collagen composition and native structure (Lomas et al. 2015; O’Brien 2005) are critical in engineering a functional implant and healing of connective tissue. Organized surface structure, high porosity, and distribution of micro- and macropores are critical attributes in bioengineered implants to support neovascularization and tissue ingrowth, maturation, and integration into the surrounding native tissue (Loh and Choong 2013; Murphy, Haugh, and O’Brien 2010; O.’Brien, Harley, and Yannas, n.d.; Han et al. 2021; Mandal and Kundu 2009; Lien, Ko, and Huang 2009; Artel et al. 2011; Druecke et al. 2003; Cao et al. 2012; Baac et al. 2004; Gnavi et al. 2015; Simitzi, Ranella, and Stratakis 2017; Gigante et al. 2009; W. Wang et al. 2016; Yin et al. 2010; Salerno et al. 2011; Hollister 2005; Gomes et al. 2006; Wei et al. 2010; Oh et al. 2014; Boyan 1996; Z. Wang et al. 2018). To support biologic healing in the subscapularis, this study utilized a collagen-based biointegrative implant that was engineered to combine aligned surface topography and porosity to mimic native tendon and aid in tendon healing. Preclinical studies in a rabbit Achilles tendon defect model showed dense collagenous fibrous connective tissue ingrowth into and around the implant (NAMSA GLP Report, n.d.). This initial pilot study was intended to further demonstrate the safety and efficacy of a utilizing a novel collagen-based implant to augment subscapularis repairs in patients undergoing TSA.
Materials & Methods
The implant selected was a collagen-based biointegrative implant, TAPESTRY® (Embody, Inc., Norfolk, VA) with a highly aligned and highly porous micro-architecture. It is composed of a co-polymer of type I collagen and poly(D,L-lactide) (PDLLA). It is manufactured using a novel fiber assembly process using electrospinning/pneumatospinning post-processing with low temperature annealing (Maghdouri-White et al. 2021).
The subscapularis tendons in five consecutively eligible patients undergoing anatomic shoulder arthroplasty for primary glenohumeral arthritis were augmented with a 50x40x25mm collagen-based implant to promote subscapularis healing (Figure 1). The patient population had a mean age of 58 (range 40 to 76). Demographics are listed in Table 1. A deltopectoral approach was utilized and the subscapularis was managed with a tenotomy or a peel technique (Figure 2). The subscapularis tendon was repaired via stem-based transosseous drill tunnels and high-tensile strength sutures for a tendon-to-tendon repair (Figure 3). Afterwards, a collagen-based biointegrative implant was secured into place over top of the subscapularis repair with an absorbable monofilament suture, slightly overlapping the enthesis on the lesser tuberosity (Figure 4). Accelerated rehabilitation protocols were utilized including a sling and limiting external rotation to 0 degrees for 4 weeks.
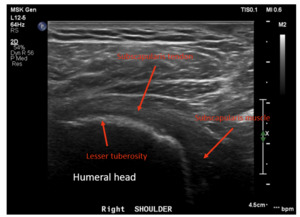
Dynamic ultrasounds performed by a fellowship trained musculoskeletal (MSK) radiologist were obtained 6 months post-surgery and evaluated for integrity of the subscapularis tendon as well as tendon thickness, width, echotexture, and calcification. Additional assessments included a standardized interview and physical examination of subscapularis function (graded belly press test and Gerber’s lift-off test), range of motion, and ASES scores were prospectively collected pre-operatively and post-operatively at 3 months and 6 months.
Results
The subscapularis tendon was intact (healed) in all shoulders as determined by the musculoskeletal radiologist. No tendon tears were observed. Four of 5 patients showed no evidence of tendinosis, while 1 patient showed mild tendinosis. Overall, the echogenic fibrillar architecture of the healed subscapularis tendon appeared normal and aligned. Six-month ultrasounds images for all patients are presented in Figures 5-9. The width of the subscapularis tendon in all patients showed a mean of 0.48 cm maximal tendon thickness and 2.72 cm mean tendon width (Table 2), similar to native tendon (Saltzman et al. 2014). The patients’ ASES shoulder scores showed an average improvement of 33 total points at 3 months and 35 points at 6 months post-operatively (MCID 13.6 +/- 2.3) (O.’Brien, Harley, and Yannas, n.d.). Results of secondary outcome measurements, including subscapularis function and range of motion, are presented in Table 3. All patients exhibited tendon healing without complications or revisions. At the 6-month ultrasound, there was no evidence of the collagen-based implant.
Conclusions
In this five patient case series, the subscapularis tendon in all patients was shown to be intact, with four showing normal fibrillar tendon architecture; one patient showed mild tendinosis and < 25% partial tear. All repairs demonstrate aligned new tissue formation rather than disorganized scar tissue. As evident in Figures 5-9, augmentation of the subscapularis tendon with the collagen-based implant was shown to be safe and effective with excellent healing outcomes at 6 months. Improvements in ASES scores, internal rotation strength and range of motion were also seen with all patients, suggesting the importance of subscapularis tendon healing after anatomic shoulder arthroplasty for optimal outcomes. This pilot study suggests subscapularis tendon augmentation has the potential to improve subscapularis healing rates and function following shoulder arthroplasty. This study is limited by a small sample size, non-randomization, and some heterogeneity in subscapularis management technique. Expansion to a multi-center, multi-surgeon clinical trial would further validate findings.
Disclosure
Amit Nathani, MD, MSc is a consultant and shareholder of Embody, Inc.’










_intac.png)











_intac.png)
