Introduction: Why
The treatment of anterior cruciate ligament (ACL) injuries have taken a fascinating journey of the past half century. Originally treated in the 1960’s via Lemaire’s anterolateral fascia lata tenodesis for “knee instability”; the rise of the arthroscope allowed a rapid progression of addressing ACL injuries. Primary ACL repair was initially popularized in the 1970’s and 80’s, however failure rates of 50% and greater led to current day movement of reconstruction using free patella tendon autografts (Feagin and Curl 1976; Gagliardi et al. 2019a). Yet along the way many evolutions and points of discussion have shaped the way we view treatment today. Debates over single tunnel verses dual tunnels, primary repair, insitu bundle augmentation, trans-tibial verses “anatomic” femoral tunnel placement, “internal bracing” augmentations, and graft choice selections are just a few of the topics that have flooded the literature in the quest to restore the most crucial of knee ligaments.
With an incidence of nearly 400,000 ACL injuries a year, ACL surgery is one of the most well known about procedures in the world pertaining to knee injuries. Success rates have historically been reported between 75% and 97% for primary surgical intervention. However short and long term issues have been associated with both ACL injuries and current surgical treatment options (Bach 2003; Baer and Harner 2007). Sequelae such as donor site morbidity, allograft failures, contralateral ACL injury due to hamstring weakness, and osteoarthritis are still known areas of imperfection associated with modern ACL surgery.
The Bridge Enhanced ACL Restoration (BEAR) procedure provides a novel alternative approach in the quest for improvement in ACL surgical treatment. The BEAR procedure allows for primary repair, or restoration, utilizing a proprietary resorbable protein-based implant for acute ACL injuries. Previous failures of primary complete ACL repairs have been attributed to a hostile healing environment of the intra-articular structure. Extra-articular ligaments, such as the medial collateral ligament (MCL), potentiate gap healing via a fibrin clot forming between the torn ligament ends. However, the intra-articular environment of the ACL has been shown to lead to premature dissolution of the fibrin clot, resulting in incomplete healing. For the BEAR procedure, the scaffold is sutured between the 2 torn ends of an ACL tear and is used to bridge the gap. The proprietary composition of the implant, coupled with specific variation of standard surgical technique, allows for maintenance of the fibrin clot at the site of healing after the implant is rehydrated with the patient’s own blood. The function of the implant further allows for a “bridge” between the torn ACL and the femoral attachment site, eliminating the need for complete re-approximation of the torn ligament (Joshi et al. 2009; Mastrangelo et al. 2011; Murray and Fleming 2013a).
How? Technical Notes for Implantation
Many of the steps for the ACL BEAR implantation procedure are unconventional for those accustomed to performing traditional ACL reconstruction. The authors have learned through experience a few technical pearls to avoid pitfalls (Table 1). A condensed surgical video of the procedure can be viewed here BEAR IMPLANT - YouTube. A tourniquet is recommended to optimize visualization as radiofrequency ablation is not advocated near the ACL stump of femoral wall. Standard arthroscopic portals are utilized for the procedure initiation. Because there is a good deal of suture management, Dr. Mc Millan advocates creating a 2 cm vertical incision at the outset for his medial portal and placing a 2cm X 10mm Arthrex Passport ™ cannula. This incision is cheated close to the medial border of the patella tendon and will also facilitate insertion of the BEAR implant later without risk of cutting the sutures. A second Passport is then placed into the lateral portal. A complete diagnostic evaluation is performed and concomitant pathology, such as meniscal tears or cartilage damage, are addressed.
Next notch and suture shuttling preparation is undertaken. Debridement of the lateral notch wall is undertaken with care taken to preserve the footprint fibers and avoidance of radiofrequency ablation. A notchplasty using a small round burr is next performed from the top of the notch, down inferior and posterior to the footprint. For traditional ACL’s the authors typically do not perform notchplasty, however for the BEAR each believes the increased bleeding will aid in healing. Femoral tunnels preparation can be done via retrograde drilling or an inside-out approach. Dr. Mc Millan has switched to a retro-drilling given the fact that a 2-3 cm incision needs to be created along the lateral femoral condyle to tie down the femoral button after suture passage. The tunnels need only be wide enough to pass 6 limbs of suture. Placement of the tunnel should be just anterior and superior to the femoral footprint to avoid damage to the native blood supply, however given the small diameter of the hole created, some surgeons have chosen to drill through the native footprint. After femoral tunnel preparation a shuttling suture is passed for later and docked out through the lateral portal. Tibial tunnel placement again can be performed via ante-grade or retro-grade drilling. Tunnel placement should be directed just anterior to the center of the footprint in order to avoid suture tangling. A shuttling suture is again placed and docked outside of the lateral portal.
Next attention is turned to the ACL stump. The length and integrity of the remaining tibial stump will dictate the method of suture passage. A trans-patellar portal can be created to allow for a tissue manipulator to be placed. In doing so, the stump can be elongated and allow for ease of suture passage through the tissue. All three surgeons prefer a self-capturing rotator cuff passer to create a total of 3 passes through the stump with each end of the suture limbs. Alternatively, a 45 degree suture shuttle can be used if the stump is rather small.
The suture used for passage through the ACL stump has been debated among many in the BEAR community. The original recommended suture is a #2 Vicryl suture. The purpose of the suture selection is that it is intended to break after 6 weeks when knee range of motion is increased and early healing has occurred. Drawbacks to this however include the lack of strength during the surgical procedure which can lead to premature suture breaking during implantation. Dr. Sigman has instead chosen to use a #2 Orthocord™ for his stump suturing, noting that it’s properties lend strength and also ability for it to resorb over time and break as desired during higher degrees of flexion.
Button Preparation and Implant Delivery
Any standard free button may be used for femoral and tibial fixation. For the femoral button, two different colored #2 Ethibond® sutures are loaded through the center two holes from top down. A separate #2 Ethibond® suture is loaded into one of the end holes and tied at the end for button shuttling. One limb from either of the ACL stump sutures are then loaded from inferior to superior through either of the center button holes and tied together at their ends as well to avoid unloading during suture passage. Next the ACL sutures and the leading Ethibond® sutures are loaded into the femoral shuttling construct and pulled up through the femur until the button exits laterally. Given the lateral incision that was made from the retro-drill, it is easy to identify the button and ensure soft tissue is not trapped between it and the bone. The ACL sutures are then clamped provisionally and tied at the conclusion of the case. Next the medial Passport™ cannula is removed and the tibial shuttling suture is brought out through this 2cm portal. The scope is next removed and the joint is prepared per protocol via irrigating with an antibiotic solution followed by drying the knee joint with suction. The 4 limbs of Ethibond® are now passed from top down through the BEAR implant. The implant can be rather stiff prior to hydration. Dr. Mc Millan has abandoned traditional a Keith needle and instead prefers the needle and loop islet cut free from a FiberLoop® suture. Each of these sutures is passed top down and spaced apart equally in the implant. The limbs are then delivered through the tibia via the shuttling suture and clamped provisionally.
Implant Hydration
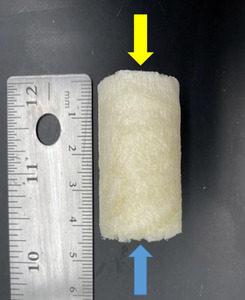
The BEAR implant (Figure 1) has a rigid, Styrofoam feel to it when removed from the packaging. Typically one end of the implant has a more firm feel than the other. This firm end should be your “base” as it will allow a firm surface when pushing the implant into the joint. 15-30 cc of whole blood will be drawn up by anesthesia when the surgeon is ready for implant hydration. The blood should be dripped over the implant from top down slowly and massaged into the sides of the implant. Avoid having the base of the implant become saturated. Place a small bowl under the implant during hydration to catch the excess run-off blood in case further hydration is required. If the implant does not begin to soften after hydration, use an 18G or larger needle to penetrate the base of the implant and inject the whole blood directly within the implant. Many physicians have asked about hydrating the implant with platelet rich plasma (PRP) or bone marrow aspirate concentrate (BMAC) however to date the effects of these biologics on outcomes have yet to be studied.
Final Implantation
The BEAR Implant is implanted into the joint blind. Just prior to implantation joint lavage is performed with antibiotic solution thoroughly. Once the implant is hydrated with whole blood, army navy retractors are placed into the 2 cm vertical medial portal incision. As the surgeon pushes the slightly still firm distal end of the implant into the joint, the leg is taken into full extension and the ACL stump sutures are pulled taught at the lateral distal femur incision. The leg does not flex after this portion of the case. With the leg in extension, the ACL stump sutures are tied over the button and the leading suture that was placed on the end hole of the button can be removed. Care should be taken not to try to make the hand ties very tight as the suture is susceptible to breaking. Mild laxity in the femoral fixation is acceptable as this fixation is provisional and protected by the rehabilitation protocol. Finally, the 4 Ethibond® sutures that were passed from the femoral button, through the BEAR implant, and out through the tibial drill hole are placed through a free button. One set of sutures can be passed through the center holes and the second set is passed through the outer holes. Each set is tied down gently and the free ends cut. The knee joint should not be irrigated any further and if the surgeon desires to place the scope back into the joint it should be done under dry conditions without flexing the knee. After closure the patient is placed into a brace locked in extension.
Discussion: When
ACL reconstruction has been performed on the premise of restoration of function and stability of the knee joint. While this has been accomplished in large part through conventional treatment options, failure rates and injury sequale have led to clinicians and patients searching for ways to improve upon the current gold standard. Considering an annual incidence of between 26.9 and 60.9 per 100,000 persons, the need to optimize the procedural outcomes is paramount as the number of new cases continue to rise. During pre-surgical discussions with an ACL patient, key components to the conversation include the risk of re-tear, risk of osteoarthritis, concomitant pathology treatment, donor site morbidity, risk of injury to the contralateral knee, and return to work or play. As this discussion unfolds the authors have found having a biologic solution for the appropriate patient candidate has particular appeal.
A substantial risk of developing osteoarthritis in the long term exist post ACL reconstruction. Approximately 50% of ACL-injured knees develop osteoarthritis within 5–15 years after the initial injury (Segawa, Omori, and Koga 2001; Daniel et al. 1994; Kessler et al. 2008). Reasons attributed to this degenerative process include the trauma from time zero injury, concomitant meniscal pathology, non-anatomic reconstruction, and graft loosening over time. In an animal model study examining ACL repairs utilizing the BEAR scaffold, macroscopic cartilage damage was significantly less than that in untreated ACL transection and bio-enhanced ACL reconstruction. Furthermore there was a strong trend (P = .068) for less macroscopic cartilage damage than in conventional ACL reconstruction in the porcine model at 12 months (Murray and Fleming 2013b). The potential for a chondral-protective benefit to ACL restoration is appealing for both surgeons and patients. Currently the BEAR III post-market clinical trial is underway and MRI follow-ups are being taken at 9 months, 2 years, 6 years, and 10 years in part to follow the development of osteoarthritis.
Graft selection and subsequent failures are also a point of conjecture when discussing the BEAR implant. Donor site morbidity (DSM) is a known issue pertaining to ACL reconstruction. Bone-patella-bone reconstructions have been associated with anterior knee pain, patella tendonitis, and in rare cases patella fracture (Kartus et al. 1997). Hamstring autografts have shown persistent knee flexion weakness, sensory nerve injury, and risk of contralateral ACL injury (Yasuda et al. 1995; Bertram et al. 2000; Arthornthurasook and Gaew-lm 1990). Quadriceps tendon autografts, while holding promise in regard DSM has still been associated with quadriceps tendonitis, quadriceps lag, and scar related issues (Mouarbes, Dagneaux, Olivier, et al., n.d.). Additionally, allograft failure rates, while improved with advances in graft processing techniques, are still reported at unacceptable rates for younger patients. In a meta-analysis performed by Ellis et al, allograft failure rates were noted to be 25% compared to 8.5% and 16.6% for bone-patella-tendon-bone a hamstring autografts respectively (Cruz et al. 2020). Of equal concern is the risk of contralateral knee ACL injuries. Spindler et al found a 12.5% risk of ACL tear in the contralateral leg within the initial 10 years after index ACL reconstruction (Magnussen et al. 2015).
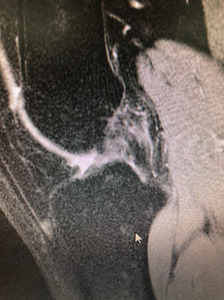
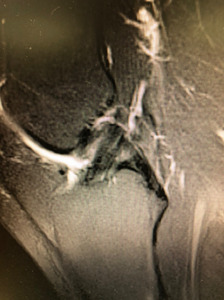
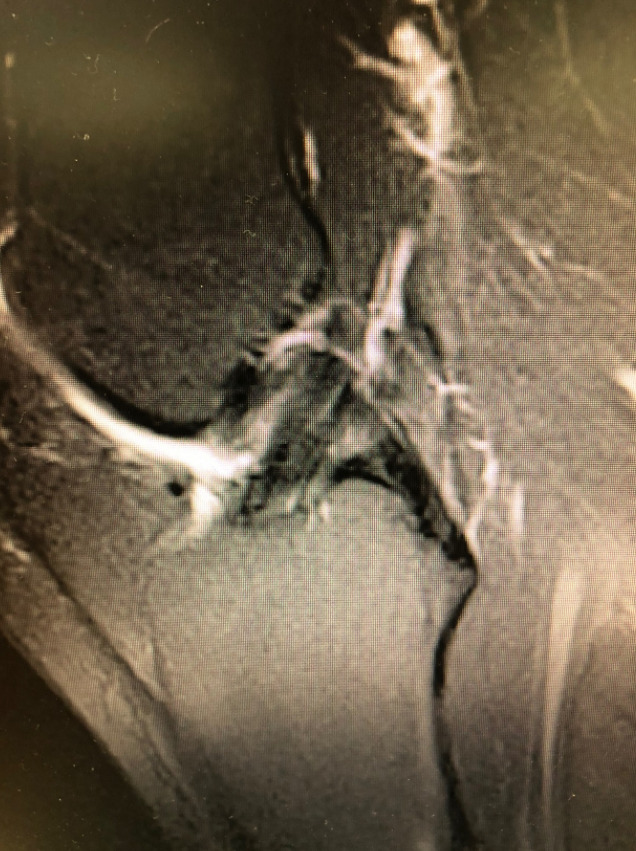
The BEAR procedure blends biology with technique with the idea of restoring native form and function for the ACL. The ability to restore the native ACL fibers and orientation with the BEAR compared to traditional ACL reconstruction has been noted through MRI follow up (Figures 2,3). Early clinical studies demonstrate similar functional hop testing when compared to traditional ACL reconstruction utilizing hamstring autograft (Murray et al. 2019, 2020). Equally as important, BEAR restoration has been shown to be non-inferior to traditional ACL reconstruction. In the first-in-human safety study reported in 2016 by Murray et al examined 2 year outcomes vs ACL reconstruction with hamstring autograft (Murray et al. 2016). These results demonstrated non-inferiority in patient-reported outcome scores (International Knee Documentation Committee [IKDC]) or significant differences in anteroposterior (AP) knee laxity between the groups. In 2020 Murray et al reported the outcome results of their Level I randomized-controlled trial examining a similar cohort of patients (Murray et al. 2020). Non-inferiority criteria were met for both the IKDC Subjective Score (BEAR, 88.9 points; ACLR, 84.8 points; mean difference, 4.1 points [95% CI, –1.5 to 9.7]) and the side-to-side difference in AP knee laxity (BEAR, 1.61 mm; ACLR, 1.77 mm; mean difference, –0.15 mm [95% CI, –1.48 to 1.17]). The BEAR group had a significantly higher mean hamstring muscle strength index than the ACLR group at 2 years (98.2% vs 63.2%; P < .001). In regards to ACL re-tears, the BEAR group reported a 14% re-tear which is well within currently acceptable range for ACL reconstruction of 10% - 28% (Astur et al. 2017; Cordasco et al. 2019; Ho et al. 2018). It furthermore is much more acceptable than the 49% failure rate that has been reported in young athletic patients (Gagliardi et al. 2019b). Interestingly, in the subgroup of BEAR ACL re-tears who were converted to traditional ACL reconstruction, their IKDC Subjective Score at 2 years similar to that of patients who had only a primary ACLR (85.5 vs 84.8 points). Furthermore, their AP knee laxity values were also similar (1.4 vs 1.8 mm). These findings are in contrast to revision of ACL reconstruction patients who often show poorer subjective and laxity scores after primary failure (Wright et al. 2011).
The ability to regenerate and restore tendons and ligaments has captivated the orthopedic field for the last decade. The use of biologics, bio-composites, and xenografts have reshaped the many of our commonly performed procedures. The ACL, given its intra-articular location, has been difficult to incorporate this philosophy, however the pursuit has not waned. The BEAR implant offers a viable alternative to traditional ACL reconstruction in the appropriate patient. The advantages of restoration rather than replacement, decreased donor site morbidity and contra-lateral knee injury, potential decrease in medial compartment osteoarthritis, and allograft failures for reconstruction hold appeal. While the procedure offers many attractive features, drawbacks still remain. The procedure is recommended to be performed within 50 days of injury for optimal outcomes. This is often not feasible due to insurance, visitation scheduling, and the need for proper pre-habilitation prior to surgery. Post-operative rehabilitation can also be a challenge. For the initial 6 week after the procedure, knee flexion and weight bearing is limited and bracing on some level is indicated for up to 12 weeks. Furthermore, the return to jogging and agility is delayed versus a traditional ACL reconstruction. Protocol understanding and follow-through on the part of the patient, provider-extenders, and the therapists is of extreme importance. The authors agree that finding the appropriate patient who they believe can be compliant with the more stringent post-operative protocol is very important to optimizing outcomes. Further research is warranted to determine wiggle room for this portion of the procedure. Return to competition is also a subject of discussion amongst the authors. Typically return to competition is not recommended in our ACL reconstruction patients prior to 9 months based on observations within the literature as well as physical examination. However exceptions are often made based upon the individual, the timing and type of sport, and other extrinsic factors. In BEAR ACL patients it is recommended to not have patients return to competition before 9 months at minimum. In taking a deeper dive into the 9 ACL restoration failures reported in Murray’s 2 year outcomes study, it was found that 6/9 (67%) of the patients suffered re-tear with un-authorized return prior to 9 months post-surgery.(S)
Having multiple tools in our bag as orthopedic surgeons is important as we continue to tailor our treatment approaches to our individualized patients. Recognizing advances in biologic consideration to aid in primary ACL repair provides hope for the treating physician and patient, similar to the hope born out the explosion of biologic augmentations in the rotator cuff. Recognizing optimal surgical candidates, grasping the nuances of the surgical procedure, and embracing the next generation rehabilitation protocols will allow for success using the BEAR ACL restoration procedure. Long term data is still being collected to validate the potential for decreased medial compartment osteoarthritis, however the early pre-clinical data is encouraging. As the procedure is now commercially available, individual surgeon preference and tweaks are inevitable to the current procedure. As longer term data is collected across all comers our understanding of why, how, and when to offer the BEAR ACL restoration procedure will become more clear.