INTRODUCTION
Degenerative rotator cuff tears are prevalent in up to 40% of middle-aged adults and in most cases lead to pain, weakness, decreased range of motion and dysfunction (Jain et al. 2014; Yamaguchi et al. 2006). As tear size increases, so does the likelihood of re-tear (Rashid et al. 2017). Repair of large rotator cuff tears requires the medialization of remaining tissue back to the tendon footprint using biocompatible sutures and anchors. Despite advances in biomaterials and arthroscopic surgical techniques over the past several decades, re-tear rate of the primary surgical repair site for large and massive full-thickness rotator cuff tears ranges from 34% to 94% (Neri, Chan, and Kwon 2009; Wu, Briggs, and Murrell 2011). Failure of surgical repair of a rotator cuff tendon injury is attributed to two main factors: tension overload at the suture tendon interface which leads to excessive tension at the repair site and insufficient biological healing of the native tendon tissue (McCarron et al. 2010; Ponce et al. 2013).
The aim of rotator cuff augmentation is to reinforce the repair construct and ultimately decrease failure. Common augments that have been used include acellular dermal matrices, collagen scaffolds, xenografts, and synthetics (Chalmers and Tashjian 2020; Steinhaus et al. 2016; Cheung, Silverio, and Sperling 2010). These augments can be broadly split into two categories: biologics and synthetics. Biologics can support healing of the native tendon by increasing tissue thickness at the implant location and serve as a source of collagen. Depending on the makeup of the biologic, the augment does not always reinforce the repair mechanically to reduce tension at the repair site. Synthetics provide mechanical reinforcement at the repair site but are insufficient in biologic healing and tissue regeneration. Therefore, neither of these independently are optimized to address the aforementioned factors that lead to rotator cuff failure.
In 2021, the BioBrace™ (Biorez, New Haven, CT), was cleared for commercial use by the US Food and Drug Administration for reinforcement of soft tissue where weakness exists. BioBrace™ is composed of a highly porous type I collagen matrix reinforced with bioresorbable poly(L-Lactide) (PLLA) microfilaments (McMillan, Arciero, and Ford 2021). This scaffold has been shown to encourage the induction, maturation, and remodeling of host tissue and load shares at time zero of implantation, thereby providing both biology and strength to the repair (McMillan, Arciero, and Ford 2021; Carter et al. 2021). These properties allow the BioBrace™ to reinforce large rotator cuff tears and provide a scaffold which supports formation of new native tissue in and around the implant as early as 3 weeks post-surgery. Furthermore, the BioBrace provides mechanical reinforcement for approximately 2 years before resorbing (Carter et al. 2021). This case report presents the use of the BioBrace™ to augment the repair of a large type-II re-tear of the rotator cuff supraspinatus tendon.
CASE REPORT
A 55-year-old female presented with pain and weakness in her right shoulder after a new traumatic event occurred. She had undergone a rotator cuff repair to this right shoulder 4 months earlier with dermal strip augmentation (Dermis on Demand™, DePuy Synthes, Rayhnem MA) secondary to poor tissue quality at the time of index surgery. For historical context, her contralateral shoulder had undergone a previous primary rotator cuff repair with xenograft, which failed, and was ultimately revised to a full thickness repair 2 years prior.
A repeat MRI demonstrated a recurrent large type-II rotator cuff tear with grade 3 Goutallier fatty infiltration.Re-tear of the primary repair of the right shoulder was confirmed on MRI one month prior to the revision surgery for the right shoulder (Figure 1).
Given the poor tissue quality noted at the initial repair, the surgeon-author consented the patient for revision rotator cuff repair with a novel 23 x 30mm biocomposite scaffold augment. The repair was performed using the double row “bridge” technique with margin convergence. Because of the crescent-shaped tear and poor tissue quality, the sutures were passed more medially with margin convergence on each side. The medial row consisted of two 5.5mm self-punching PEEK anchors loaded with #2 Dynacord™ suture. (Healix Advance BR PEEK, DepuySynthes, Rayhnem MA). An Expressew III suture passer (DePuySynthes, Rayhnem MA) was used to arthroscopically pass sutures through the tendon and those same sutures were passed through the medial aspect of the BioBrace outside the body. The graft is oriented with the long side running medial (glenoid side) to lateral (tuberosity side) to maximize the structural strength of the implant. Knots were tied on the anteromedial and posteromedial corners of the BioBrace™ to secure the medial border of the scaffold. Those sutures were then passed through 2 PEEK self-punching lateral anchors to complete the double row repair construct (Figure 2A, 2B). After discharge, the patient followed the surgeon-author’s standard of care physical therapy regimen for arthroscopic rotator cuff repair consisting of a sling for 2 weeks followed by gradual assisted and active assisted range of motion progression beginning at week 3. Periscapular strengthening was begun at 6 weeks and progression to unrestricted activity was permitted after 6 months following the revision procedure.
The patient was seen at three, eight, 10, and 12 months after surgery. Review of MR images at three months indicated the rotator cuff was extending across the entire footprint with robust tendon thickness and some fluid in the subacromial space (Figure 3A). MR imaging at 8 months demonstrated maturation of tendon healing and no further fluid within the subacromial space (Figure 3B). This indicates functional tissue remodeling over time.
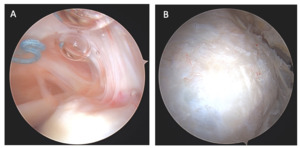
At 10 months post-operatively, the patient presented with residual bicipital pain. Conservative care was attempted and failed. Based upon this the patient underwent second look arthroscopy and a biceps tenodesis. Intra-articular images were taken of the rotator cuff during this procedure and can be seen below in Figure 4. On the articular side of the rotator cuff, the visible, prominent suture was identified and easily removed (Figure 4A). Tendon fibers can be seen inserting on to the footprint in an organized fashion.
On the bursal side of the rotator cuff, images taken at 10 months post-operatively indicated that BioBrace™ scaffold was well-synovialized and completely incorporated into the rotator cuff, with new native tissue in and around the implant.
RESULTS
The most recent follow-up, 1 year after the index procedure, confirmed that the patient has achieved full range of motion with minimal complaints of pain and discomfort, and 4/5 strength of the supraspinatus. The patient reported minimal stiffness and minimal “quick pain” during certain planes of movement. She was continuing a strengthening program due to her recent biceps tenodesis procedure.
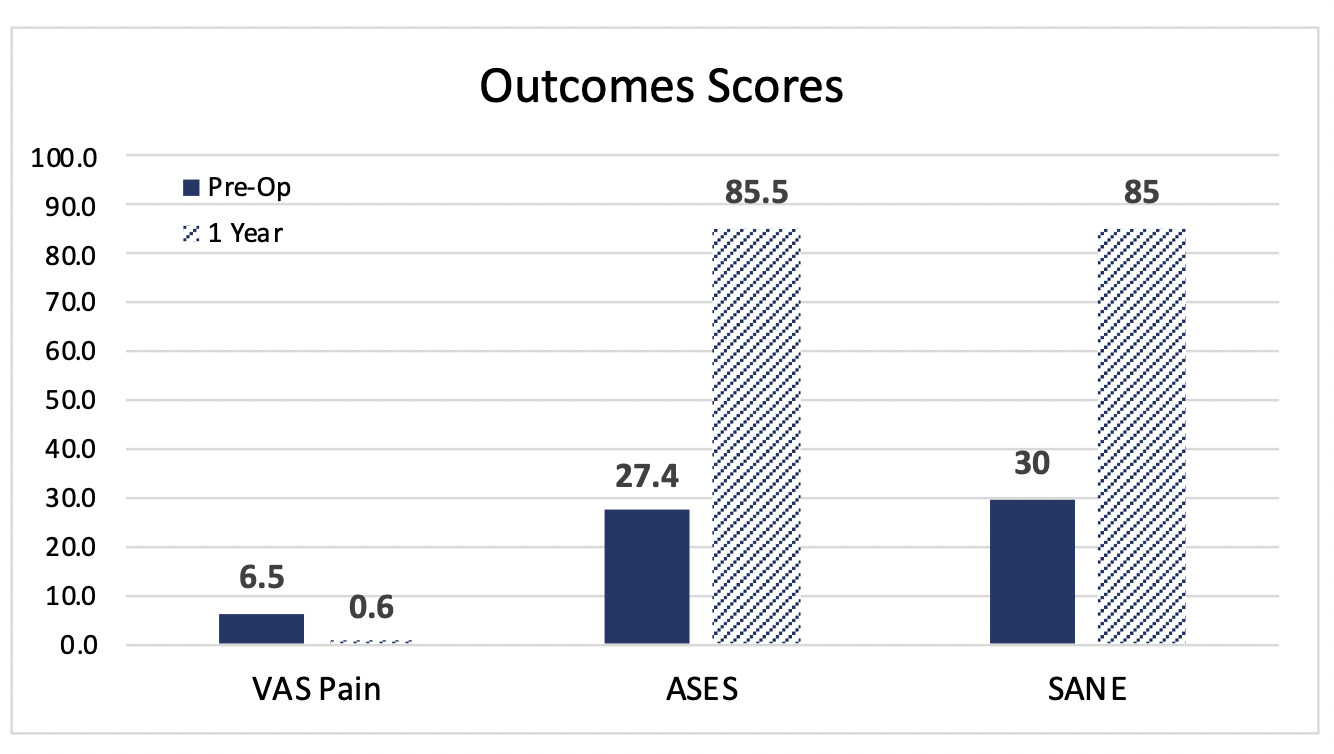
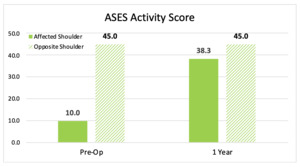
Patient reported outcomes using Visual Analog Scale (VAS), American Shoulder and Elbow Surgeons (ASES) Shoulder Score, and Single Assessment Numeric Evaluation (SANE) were assessed before the index procedure and at 1-year follow-up. Shoulder pain and function showed marked improvement over the 1-year postoperative period. ASES activity scores were used to compare the affected shoulder to the opposite shoulder (Figure 5). The change in scores over time demonstrates that the operated shoulder is nearing the activity level of the opposite shoulder. When comparing preoperative vs. postoperative scores, the VAS pain score, ASES, and SANE all showed improvement at the 1-year follow-up point (Figure 6).
DISCUSSION
Re-tear rates of large type-II rotator cuff repairs remain high (Neri, Chan, and Kwon 2009; Wu, Briggs, and Murrell 2011). 62-78% of rotator cuff failures occur in the first three months after surgery (Bushnell et al. 2022; Kluger et al. 2011; Miller et al. 2011). Early re-tears (within 3-6 months) are primarily attributed to mechanical failure, while re-tears that occur later on are due to a lack in biologic healing at the bone-tendon interface (Wu, Briggs, and Murrell 2011; Miller et al. 2011). Rotator cuff repairs that fail by 3 months indicate a failure to heal rather than a re-tear after healing (Bushnell et al. 2022; Kluger et al. 2011). This suggests that repairs must be protected and reinforced mechanically during the early healing stages, when the tendon is weakest and most susceptible to failure. The most common mode of failure in rotator cuff repairs is suture pull through at the suture-tendon interface (Ponce et al. 2013; Mirzayan et al. 2019).
Kwon et al. published a 15 point scoring system aimed at determining rotator cuff healing rates after surgical repair (Kwon et al. 2019). This system awarded points based up retraction, fatty infiltration of the infraspinatous, age, AP tear size, bone mineral density (BMD), and work activity. Patients with ≤4 points accounted for 6.0% of the identified healing failure rate, and those with ≥5 had a 55.2% healing rate and patients with a score ≥10 points demonstrated an 86.2% healing failure rate. The patient presented in this case report had a minimum score of 8 without knowing her BMD, placing her at a high risk of recurrent tear.
Inherently it would make sense that a rotator cuff repair would benefit from structural support and mechanical strength at time zero in order to prevent re-tears and suture pull through, particularly through the first 3-6 months. Re-tears can also be decreased by increasing tendon thickness via the induction of new host tissue, as this reduces the strain on the native tendon and restores the bone-tendon interface (Thon et al. 2019; Bokor et al. 2019).
Current treatment options aim to restore rotator cuff tissue back to its native footprint. Large and massive tears of the rotator cuff are surgically repaired using biocompatible suture anchors. These repairs can be augmented with synthetic or biologic products to reinforce repairs and reduce risk of failure, but to date, there is no single implant that supports healing while providing mechanical reinforcement.
A wide variety of biologic and synthetic augmentation materials have been reported in the literature as methods to improve healing and durability of the rotator cuff repair (Chalmers and Tashjian 2020; Steinhaus et al. 2016; Cheung, Silverio, and Sperling 2010). Augmenting the repair will allow for load sharing at the suture tendon interface to offload tension at the repair site while providing biology to support healing of native tendon tissue. While existing augments have met with some success, there remain concerns to be able to optimize repairs with them. Synthetic materials and acellular dermal matrices are associated with a lack of biological integration (Lederman et al. 2021; Adams et al. 2006).
BioBrace™ provides strength at time zero to protect the repair from early re-tears and infers a mechanical advantage to prevent suture pull-out. The use of this biocomposite scaffold could potentially mitigate the risk of re-tears in the immediate post-operative period, when failure rates are known to be high (Bushnell et al. 2022; Kluger et al. 2011; Miller et al. 2011). Furthermore, this biocomposite scaffold promotes healing by allowing for the induction and remodeling of new host tissue and increasing tendon thickness, before ultimately resorbing (Carter et al. 2021).
This patient presented a challenge due to poor tissue quality and the large size of the re-tear. The repair was augmented with BioBrace™ scaffold to encourage the induction, maturation, and remodeling of host tissue and load share at time zero of implantation, thereby providing both biology and strength to the repair. Postoperative MR images at eight months and arthroscopic images taken at 10 months (second look due to residual biceps pain) indicated that the BioBrace™ was fully incorporated with new native tissue in and around the implant. VAS pain, ASES, and SANE scores were all noted to be improved and were higher than what is typically expected for this type of case within the literature (Kim et al. 2020; Cvetanovich et al. 2019). Furthermore, the ASES activity score of her operated shoulder is nearing the activity score of the opposite shoulder, suggesting near normal function. Post-operative MR images and results from the most recent follow-up indicate that the patient is on track towards a full recovery. Limitations of this manuscript exist given that it is a case report. MCID for ASES cannot be accurately assessed in this scenario. Longer term follow up of 2 years minimum should ideally be performed to assess the continuation of the early positive outcomes seen. Furthermore, there is a lack of generalizable data on the effectiveness and cost-benefit of biologics in primary or revision rotator cuff repair. Nevertheless, this case report is promising and provides a potential alternative to traditional dermal grafting for patients with poor tissue quality, particularly in the revision setting. Further studies with more patients and longer-term follow-up are required to better evaluate the potential clinical benefits of this implant.



_and_8_months_(b).jpg)



_and_8_months_(b).jpg)