This is the third of four award-winning presentations from the inaugural Medical School Orthopedic Society (MSOS) symposium. MSOS is a medical student-run initiative with a mission to support “research and educational opportunities for students interested in orthopedic surgery.”
This presentation was given by Lindsey Johnson, a rising fourth-year medical student at Campbell University who recently completed an orthopedic surgery research year at Duke University.

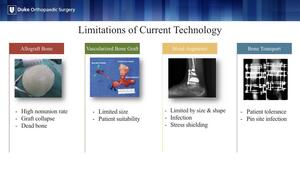
One of the unmet needs in orthopedic surgery is the critical-sized bone defect. These defects can be the result of congenital deformity, high energy trauma, non-union, failed arthrodesis or arthroplasty or the degenerative pathology we see in Charcot.
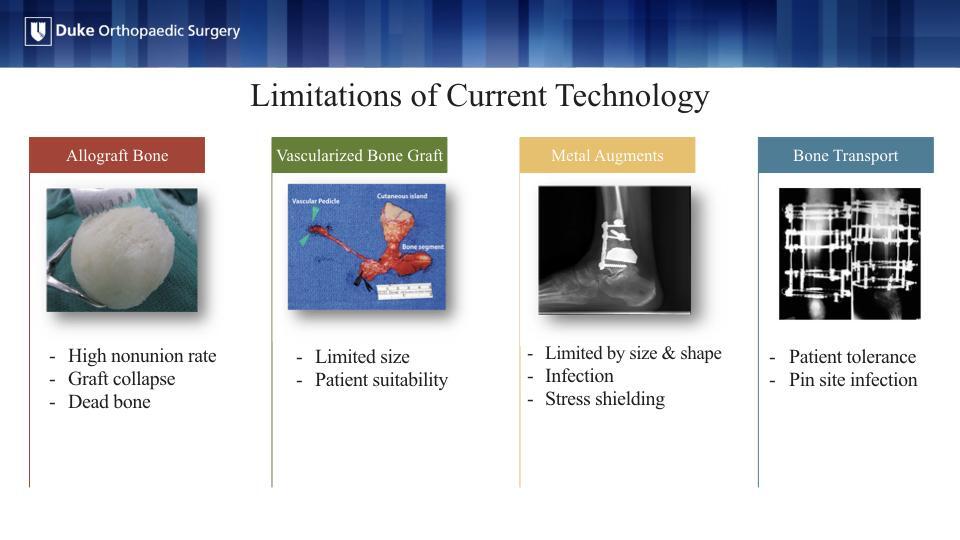
The current treatment options for these bone voids are limited in their size, risk of rejection or infection. They often require multiple operations that are burdensome for the patient.

3D-printing has partially addressed these concerns through patient-specific implants often made of titanium or cobalt chrome, but these are still foreign materials that are subject to the same risk of infection and non-union.

In our study, we described the suitability of an extrusion-based 3D bioink composed of gelatin and gelatin methacryloyl with various concentrations of hydroxyapatite (HA). Osteoblasts were added to this bioink formula for bone tissue engineering. We hypothesized that the addition of HA would (1) decrease hydrogel swelling and degradation and (2) increase osteoblast cell proliferation and markers of bone matrix deposition.

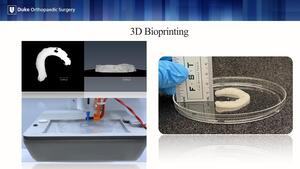
This is where bioprinting comes into play! The ultimate goal is that the bioprinted construct will be implanted in the body, but ultimately degrade as the patient’s bone grows in its place.
A micro-CT image is created often from the contralateral limb. This file is translated into a form readable by the bioprinter, which lays down a layer-by-layer construct from the bioink components. The printed construct then undergoes a crosslinking process for stability and is placed into osteogenic culture media to allow the cells to grow into the hydrogel.

We printed our hydrogel construct in a porous cube with the respective components previously described. The construct then underwent crosslinking via photopolymerization under UV light, which was then cultured in media for 1, 14 and 28 days. After which we performed a swelling and degradation analysis, cell viability and proliferation assay, and routine histology.
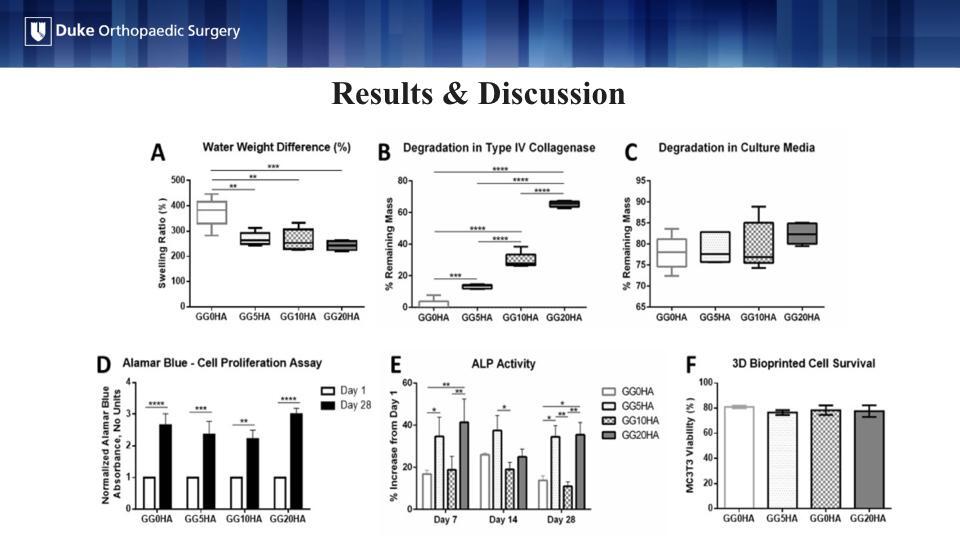
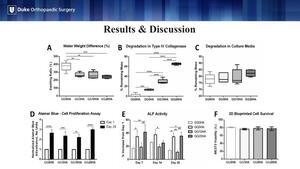
The addition of HA at any concentration significantly reduced hydrogel swelling in comparison to the same hydrogel without HA (Figure A). This is clinically relevant to future in vivo implantation as minimizing hydrogel swelling would help ensure the construct does not significantly change size when implanted in the body. HA significantly decreased hydrogel breakdown in the presence of type IV collagenase, an enzyme present in the body that serves as surrogate for how quickly the construct would degrade in the body (Figure B).
The results in Figure C are critical to the overall tissue engineering process. Printed constructs will require a time in media before implantation to allow the cells to proliferate. However, HA did not decrease hydrogel degradation as was seen with collagenase (Figure C). A significant increase in cell proliferation at day 28 was noted in all groups, indicating that HA was not cytotoxic at any concentration (AlamarBlue) (Figure D).
Alkaline phosphatase (ALP) activity is an early marker of osteogenic differentiation. In our study, ALP activity significantly increased with the addition of 5 mg/mL and 20 mg/mL of HA at days 7 and 28. This suggests that HA plays a role in osteoblast differentiation and mineralization (Figure E). Figure F shows the results of post-printing cellular surviving, indicating that the cells could withstand the stress of the printing process (Figure F).
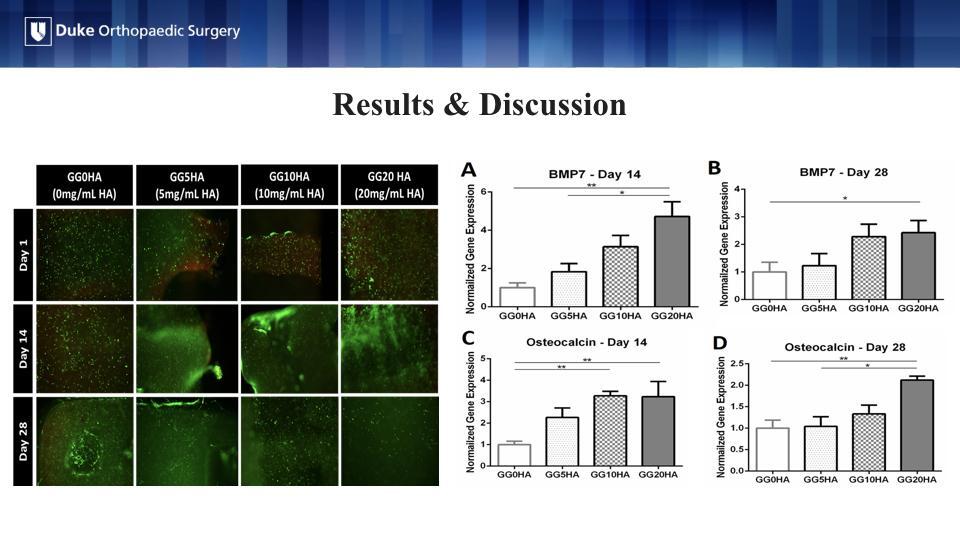
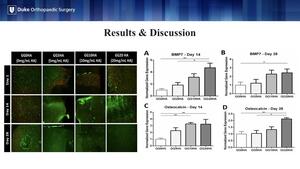
Live/dead staining on the left visually confirms the results that the cells were alive at each time point, post-printing with the green cells being the live ones and the red being the dead cells.
Finally, we looked at levels of gene expression of BMP-7 and Osteocalcin, a bone protein and an osteoblast-derived hormone, respectively. In all cases, the addition of 20 mg/mL of HA resulted in significantly greater gene expression of BMP-7 and Osteocalcin over hydrogels without HA. This indicates 20 mg/mL is the lowest threshold HA concentration to support osteogenic gene expression.

The addition of HA to GelMA-gelatin hydrogels (1) decreased hydrogel swelling, (2) improved resistance to enzymatic degradation, (3) increased osteoblastic differentiation and mineralization, (4) increased osteogenic gene expression, and (5) maintained equal cell viability and proliferation. These are some of the first steps toward a viable, 3D bioprinted implant.
Disclosures
The authors do not have anything to disclose pertaining to this presentation.