INTRODUCTION
Postoperative pain management in joint arthroplasty is an ongoing and relevant issue. Pain after lower extremity total joint arthroplasty has traditionally been managed with high doses of narcotic pain medications (Bonica 1990). Total ankle arthroplasty (TAA) is a particularly painful procedure, often controlled with narcotics, which have been shown to lead to respiratory distress, postoperative ileus, increased hospital stay and decreased patient satisfaction (Bonica 1990; Chou et al. 2008; Zhao et al. 2004).
In an effort to decrease postoperative dependency on narcotic medications, a regional nerve block at the level of the knee is frequently used as an adjunct procedure for postoperative pain control, with its use ubiquitous within foot and ankle surgery (Hansen, Eshelman, and Cracchiolo 2000; Young, Cota, and Chaytor 2014). Peripheral nerve block (PNB) has the advantage of excellent pain control for approximately 10-18 hours after the surgery. In addition, it has been shown to provide equivalent postoperative pain relief to general anesthetic alone (Goldstein et al. 2012). While PNBs at the popliteal level are considered the gold standard for many foot and ankle procedures, there are risks and drawbacks. One disadvantage to a “single shot” regional nerve block at the region of the popliteal fossa is “rebound pain” which can occur between 10-24 hours after the procedure (Goldstein et al. 2012; Ilfeld et al. 2002). Additional minor complications can occur when using a continuous catheter, including drug leakage, failure of the infusion device, catheter dislodgement, catheter block and incomplete anesthetic coverage (Ma et al. 2019). PNB placement requires an additional procedure, potentially delaying the procedure start time, and adding additional resources and financial cost. To decrease the incidence of postoperative dysesthesia while still decreasing opioid usage after lower extremity surgery, alternatives to regional nerve blocks have been explored.
In total hip and knee arthroplasty, intraoperative multimodal periarticular injections have been increasingly used in recent years (Lombardi et al. 2004). These injections have been found to decrease postoperative pain and increase patient reported outcome measures without the need for additional regional neuraxial anesthesia (Kerr and Kohan 2008; Parvataneni et al. 2007). Recently, periarticular multimodal analgesia has been described in other lower extremity surgery including tibial osteotomy and the surgical treatment of ankle fractures (Hancock et al. 2019; Y. S. Kim et al. 2016). Mulligan et al. (2017) compared pain scores, need for prolonged narcotics, and complications between liposomal bupivacaine injections versus PNB in a retrospective chart review of TAA patients, finding no differences between pain control modalities (Mulligan et al. 2017). However, little is known about the efficacy of periarticular (PAI) joint injections containing a “cocktail” of drugs targeting postoperative pain management in TAAs. Given the benefits described in total knee and hip arthroplasty, we hypothesized a similar benefit would be seen with TAA. To test our hypothesis, we designed a randomized, prospective trial to evaluate narcotic use and self-reported pain in the first 24 hours and discharge through two weeks following TAA, and captured complications through three months postoperatively.
METHODS
Participants
All opioid naïve patients between 40 and 80 years old presenting to a single, fellowship-trained foot and ankle surgeon for an ipsilateral, primary total ankle arthroplasty, were identified and screened for study participation between 2016 and 2020. Exclusion criteria consisted of a poor social situation which would make follow-up or tracking pain medications difficult; uncontrolled endocrine disease; uncontrolled diabetes or neuropathy; serious heart or vascular disease; active cancer; narcotic use preoperatively consisting of either a transdermal patch or pain stimulator; and planned discharge to a skilled nursing facility.
Study Design
We conducted a prospective, randomized, non-inferiority study with two parallel arms in which participants were selected to either receive a periarticular multimodal injection (PPAI) or a preoperative peripheral nerve block (PNB), defining PNB as the comparator. A non-inferiority trial was performed as it is not known if PAI would be more effective than PNB for postoperative pain control; however, PAI may eliminate the risk associated with nerve blocks. If PPAI is no worse than PNB, there may be a clinical rationale to adopt PPAI as the standard of care for the management of immediate postoperative pain for TAA. Randomization was performed at the study initiation by using a computer-generated list for 1:1 allocation of PPAI versus PNB stratified by patient sex (Stata version 14; StataCorp, LLC, College Station, TX). Data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at our institution (REDCap, (Harris et al. 2019, 2009). All participants provided written informed consent. This protocol was approved by the Institutional Review Board and registered with ClinicalTrials.gov.
Surgical Technique and Treatment Arms
Each study participant underwent a TAA either through a standard anterior or lateral approach. For participants randomized to PAI, after finalization of the implantation the wound was irrigated. A sterile syringe was used to administer the cocktail, mixed by the pharmacy, consistent with PAI for total knee and hip replacement at our institution. The periarticular ankle block was performed at the completion of the procedure with 50cc containing 125 mg of ropivacaine, 15 mg of ketorolac, and 40 mcg of clonidine diluted with 48 ml of normal saline. Prior to closure, 12.5 ml was injected into the posterolateral ankle recess, 12.5 ml into the medial and lateral gutter and collateral ligaments and the remainder was injected into the anterior joint capsule. In a lateral approach total ankle arthroplasty, the “cocktail” is injected into the posterior capsule/retrocalcaneal space, next into the medial joint/capsule, then anteriorly, and the remaining injected into the lateral capsule. All incisions were then closed with non-absorbable sutures in the skin layer and the ankle was then splinted for two weeks.
Participants randomized to receive the preoperative regional anesthesia underwent PNB in the preoperative holding area. PNB included a single popliteal fossa block of the sciatic nerve and an adductor canal block of the distal femoral nerve administered by the anesthesiologist. As the PNB is performed by the anesthesiologist prior to induction under ultrasound guidance, it was not possible for patient blinding. A single shot injection at the popliteal fossa was used per standard of care while the patient was positioned in either the lateral or prone position according to the anesthesiologist’s preference. The anesthesiologist used a 4.0 inch needle. For all blocks, 0.25% bupivacaine was used for the saphenous and popliteal nerves. Block success was evaluated by anesthesia documentation of patient satisfaction and comfort (i.e., loss of sensation).
Postoperatively, patients were given 5 mg to 20 mg of oxycodone orally every three hours as needed for moderate to severe pain (at least 4/10 on 0-10 scale by patient self-report). In addition, intravenous hydromorphone (0.5 mg) was offered for breakthrough pain. Patients who had an allergic reaction or intolerance to oxycodone were changed to an alternative oral opioid.
Outcome Measures
The primary outcomes were mean difference in postoperative narcotic consumption and postoperative pain, respectively, assessed at 24 hours and two weeks postoperatively. Narcotic consumption was assessed by inpatient record and daily narcotic diaries completed by the patient at home after discharge. All narcotics were standardized to morphine milligram equivalents (MME) and a total for each patient was calculated at 24 hours (inpatient) and cumulatively from hospital discharge through two weeks postoperatively, which were consistent benchmarks among a population with a length of stay varying from postoperative day one to postoperative day two. Discontinuation of narcotics before two weeks postoperatively, captured as yes/no, was also evaluated. Postoperative pain was assessed using the Visual Analogue Scale (VAS) for pain, a sliding bar scale which allows the patient to identify current pain level from no pain (0) to worst possible pain (10) (Huskisson 1974). Participants were asked to place a single mark on a 100 millimeter line.
Participant demographic data included age at the time of surgery, sex, race and ethnicity, insurance type, smoking status, alcohol and drug use history, self-reported assessment of physical activity, all of which were collected at the preoperative appointment. Baseline assessments included administration of the Patient Health Questionnaire-9 (PHQ-9), a depression index scale (Kroenke, Spitzer, and Williams 2001); the Veterans RAND 36-Item Health Survey, a measure of health-related quality of life, reported as physical health component and mental health component scores (Kazis et al. 2004); and the Foot and Ankle Ability Measure (FAAM) Activities of Daily Living Subscales, which is a patient reported outcome of joint-specific disability (Martin et al. 2005). Operative data included approach, additional procedures performed simultaneously, procedure time, implant manufacturer, and length of stay. Postoperative complications possibly associated with TAA, PNB (i.e., persistent numbness), and PPAI were evaluated during admission, and at two weeks, six weeks, and three months postoperatively.
Statistical Methods
Demographic and baseline characteristics were compared between study groups to evaluate adequacy of the randomization and comparability across cohort characteristics. Fisher’s exact tests were used to compare categorical data. Continuous data were evaluated for normality. The means of normally distributed continuous variables were compared using a t-test after evaluating variance equality. Non-parametric approaches were utilized to compare characteristics and outcomes between study groups for skewed data. Means and medians with interquartile ranges (IQR) were reported for outcome measures.
Data analysis for the primary outcomes of interest, postoperative MME consumed and postoperative pain (VAS), was used to determine if the mean difference between groups was within the defined non-inferiority margins. These margins were set to 30 MME (24-hour POD1) and 75 MME (discharge through two weeks) for the mean difference in narcotic consumption and 15mm on the VAS, using the upper limit of one-sided 95% confidence intervals (UCI) for increase in MME and VAS score to judge non-inferiority.
The non-inferiority limit for 24-hour opioid consumption in studies in which MME consumption was the primary outcome previously used 30 MME as an established limit (Lee et al. 2018). Since standards for opioid consumption following TAA have not been established, the 2-week threshold was set to 75 MME, which is the MME in our institution’s single narcotic refill protocol following TAA (one refill after discharge— prescription for 30 tablets of oxycodone 5mg).
The threshold of 15mm on the VAS was selected by reviewing studies evaluating the minimal clinically important difference (MCID) and published non-inferiority margins. In joint arthroplasty, the minimum detectable change for total hip arthroplasty has been reported at 14.9mm and knee arthroplasty at 16.1mm (Danoff et al. 2018). Prior TKA PNB versus PAI studies have used 13mm as the non-inferiority threshold (Kertkiatkachorn et al. 2021), while other studies evaluating postoperative pain control following orthopedic procedures have used 10mm (Darrieutort-Laffite et al. 2019) to 15mm (H. J. Kim et al. 2017). A recent systematic review concluded that a clinically relevant MCID for VAS after hip and knee arthroplasty is 15mm (Laigaard et al. 2021).
This study was not initially powered as a non-inferiority trial. A power analysis for non-inferiority was performed prior to data analysis. Power was evaluated based on a published MME mean for a recent similar trial in total knee arthroplasty within the adductor canal block treatment arm; 24 hour (POD 1) opioid consumption had a mean of 48 MME with a standard deviation of 25 MME (Grosso et al. 2018), and an MCID of 30 MME (non-inferiority limit). A total of 24 patients were needed to achieve 80% power at a one-sided significance level of 0.025.
RESULTS
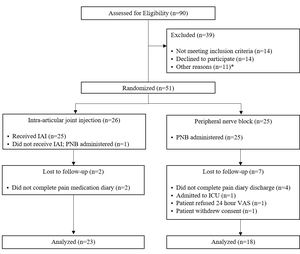
Between 2016 and 2020, 90 consecutive TAA patients were screened for eligibility and 51 were randomized to PAI (n=26) or PNB (n=25) (Figure 1). Patients who did not have complete VAS or MME outcome data due to lack of compliance (n=6) and patient refusal/withdrawal (n=3), were excluded from analysis. The final cohort contained 23 PAI and 18 PNB participants. All patients randomized to PNB had successful blocks.
There were no significant differences in demographic or baseline characteristics between study groups, with two exceptions (Table 1). Participants randomized to PNB self-reported a higher PHQ-9 depression index score compared to participants randomized to PAI; 44.4% of PNB participants had at least “mild” depression compared to 17.4% in the PAI group (p = .034). Additionally, participants in the PNB group reported significantly lower subjective ankle function on the FAAM (p = .039), with those in the PNB group reporting a mean percent function of 32.7 (sd = 21.5) compared to the PAI group—49.5 (sd = 24.6), despite no significant differences on the FAAM Activities of Daily Living Subscale (p = .699). The majority of the study population was male (72% PNB and 65% PAI), with a primary diagnosis of posttraumatic arthritis (67% PNB and 65% PAI), and obesity (mean BMI ≥ 30). There were two current cigarette smokers in the study, and both were randomized to the PNB group, while four participants in each group reported current marijuana use.
Table 2 compares the surgical characteristics between study groups. There were no significant differences in surgical approach, manufacturer, count of additional procedures, procedure time, or length of stay between patients randomized to receive PNB compared to PAI. The majority of patients in the PAI and PNB groups had TAA procedures with an anterior approach using a Wright Medical implant. A higher proportion of PNB patients had additional procedures (83%) compared to PAI (65%), although these proportions were not significantly different (p = .291). Patients were admitted for an average of 1.67 days (sd = 0.59) in the PNB group compared to 1.78 days (sd = 0.60) in the PAI group (p = .643) after similar procedure time (p = .474).
There were no significant differences in MME consumed or VAS pain score in the first 24 hours or discharge through two weeks, respectively. Among the entire cohort, MME consumed in the first 24 hours ranged from zero to 254 MME, with a mean of 57.9 (sd = 51.2) and a median of 43.5 (IQR, 22.5-76.0). The variability in narcotic consumption from discharge through two weeks postoperatively was high in both groups. Mean narcotic consumption was 346.8 MME (sd = 314.6) in the PNB group with a median of 261.3 MME (IQR, 82.5-525.0). A higher mean MME was observed in the PAI group (427.6, sd = 522.1) with a median of MME 210.0 (IQR, 100.0-585.0).
Self-reported pain on the VAS was consistent between groups at 24 hours postoperatively (p = .991). The PNB group reported 25.2mm (sd = 29.4) with a median of 15.0 mm (IQR 1.0-46.0). The PAI group reported 25.1mm on the VAS (sd = 24.7) with a median of 14.0mm (IQR, 3.0-51.0). Self-reported pain decreased to a mean of 16.4mm (sd = 21.0) and 14.6mm (sd = 17.9) among PNB and PAI Groups at two weeks postoperatively, respectively, with a median of 7.5mm (IQR, 1.0-26.0) in the PNB group and 9.0mm in the PAI group (IQR, 0.0 – 18.0) (p = .828).
Days until narcotic discontinuation trended toward a reduction in narcotic days in the PAI group compared to the PNB group; however, the difference was not statistically significant (p = .155). The PAI group stopped taking narcotics, on average, 19.1 days (sd = 23.6) after surgery, while the PNB group continued to an average of 25.6 days (sd = 16.1). There were more patients who trended toward longer narcotic use in the PNB group as indicated by a median 15.5 days with the upper 75% quartile at 31.0 compared to median 11.0 with an upper IQR of 16.0 in the PAI group.
There was no significant difference in the proportion of patients with complications at three months post-TAA in the PNB versus the PAI group (p = 1.000). Three patients in each group had one complication (16.7% PNB, 13.0% PAI). Complications included one reoperation (5.6% PNB), three cases of persistent numbness/dysesthesia (5.6% PNB versus 8.7% PAI), and two cases with unresolved pain beyond what would be expected at three months (5.6% PNB versus 4.4% PAI) (p = .879).
The non-inferiority margin for mean difference in narcotics consumed at 24 hours was 30 MME (Figure 2). The upper confidence interval (UCI) observed was 32.6 MME, crossing the non-inferiority margin by 2.6 MME. The variability observed for MME consumed between discharge and two weeks postoperatively was large, with the 95% confidence interval on the mean difference of 80.0 ranging from -201.6 to 363.3, with the UCI greatly exceeding the non-inferiority margin set to 75.0 MME. The mean difference in the pain VAS reported at two weeks was 0.09 with a UCI of 17.0, which exceeds the non-inferiority margin set to 15mm by 2mm. However, at two weeks postoperatively, the mean difference was -1.9mm with a UCI of 10.4mm, which is well under the 15mm non-inferiority margin. A post-hoc analysis using the observed standard deviation of 51.2 MME for the 41 enrolled patients yielded an under-powered result of 57% at a one-sided significance level of 0.025.
DISCUSSION
The use of multimodal surgical site injections has become increasingly popular in lower extremity surgery, including elective hip and knee arthroplasty, trauma and sports medicine (Y. S. Kim et al. 2016; Hancock et al. 2019; Fu et al. 2009; Parvataneni et al. 2007). Previous studies have shown periarticular multimodal injection to be equivalent to regional anesthesia for hip and knee arthroplasty (Li et al. 2018). Our study showed no significant difference in mean MME consumption or VAS pain scores at 24 hours and two weeks postoperatively when comparing multimodal periarticular injection to that of a regional popliteal block. However, this study did not support the non-inferiority of PAI relative to PNB for 24-hour postoperative pain or narcotic consumption with UCIs breaching non-inferiority margins specified by approximately 2mm on the VAS and 2.6 MME, respectively. At two weeks post-TAA, PAI did demonstrate non-inferiority for the 2-week VAS, but high variability in postoperative narcotic consumption breached conservative UCIs for the non-inferiority margin.
In foot and ankle surgery, a dual-catheter regional block with continual saphenous and popliteal infusion has performed better that a popliteal catheter single injection saphenous block for opioid consumption and pain scores on postoperative days one through three (Jarrell et al. 2018). However, peripheral nerve blocks are not without risks or complications. Some of the drawbacks to peripheral nerve blocks previously reported consist of rebound pain (Goldstein et al. 2012), loss of motor function, procedural time and cost. While previous reports have described nerve injury as rare (Ma et al. 2019), other prospective studies have shown that they can be as high as 24% (Brull et al. 2007; Anderson et al. 2015; Gartke, Portner, and Taljaard 2012).
Use of a periarticular injection in total joint arthroplasty has been described as an effort to provide pain relief without affecting motor function. While still providing adequate analgesia, use of a periarticular block in hip and knee arthroplasty has been found to decrease procedure time and expense as well as decreasing nausea and improving range of motion (Li et al. 2018; Parvataneni et al. 2007; Kerr and Kohan 2008). For a number of years, our institution has utilized a multimodal approach in hip and knee arthroplasty patients, including periarticular injection consisting of ropivacaine, clonidine and Toradol as an alternative to a peripheral nerve block.
Multimodal periarticular injection has been previously described as effective for pain relief in foot and ankle surgery. Kim et al. described the use of multimodal injection for postoperative pain relief in the foot and ankle (B. S. Kim et al. 2011). They performed 61 bilateral bunion corrections and injected the cocktail into one foot while the other was treated with a saline injection only. They found that the side with the multimodal injection resulted in decreased pain and higher patient satisfaction. Other studies have confirmed that multimodal injection can be effective for pain relief in a variety of foot and ankle procedures including supramalleolar osteotomies and ankle fractures (Turan et al. 2007; Y. S. Kim et al. 2016; Hancock et al. 2019). However, all of these compared a multimodal injection to a placebo. To our knowledge, there are no previous studies comparing multimodal periarticular injection to a peripheral nerve block for pain control after total ankle arthroplasty.
Exparel® has also been described as an alternative to peripheral nerve blocks. Exparel® (liposomal bupivacaine) is a liposomal formulation of bupivacaine approved by the Federal Drug Administration for injection into surgical wounds for perioperative pain relief. The medication allows for the slow, extended release of bupivacaine for up to 72 hours after injection by using DepoFoam as the delivery system. Liposomal bupivacaine has been used in a variety of orthopaedic surgeries including hip, knee and shoulder replacement with some success (Jain et al. 2016; Bagsby, Ireland, and Meneghini 2014; Cherian et al. 2016). It has also been used for pain control in bunion and forefoot surgery (Huh and Parekh 2014). Mulligan et al. (2017) performed a Level III head-to-head study comparing the addition of a liposomal bupivacaine single shot continuous popliteal sciatic nerve block with 0.2% ropivacaine, versus 0.2% ropivacaine nerve block with an indwelling catheter alone in patients undergoing a TAA. They saw no differences in terms of complications, ED visits, reoperations, VAS pain scores or narcotic refills. They determined that liposomal bupivacaine was a safe and effective addition to regional anesthesia for postoperative pain control and rendered catheter maintenance unnecessary (Mulligan et al. 2017).
Our community standard for foot and ankle surgery has consisted of a single shot popliteal block with intermittent medial coverage using an adductor canal block to cover the saphenous nerve. Intermittently, we use a catheter to provide continuous coverage but have found that issues with cost, time for catheter placement and medication leakage are barriers to patient satisfaction. Thus, for this study, we elected to compare the intra-articular injection to a single shot popliteal injection with an adductor block of the saphenous nerve in an effort to directly compare it to our current practice. Future studies could also be performed comparing the effects of intra-articular injection to that of a continuous catheter infusion or liposomal bupivacaine.
One important strength of our study is the prospective and randomized design. Inclusion of patient tracking logs facilitated an accurate accounting of amount and duration of opioids consumed after discharge. Post-hoc power calculations suggest the current study may be underpowered for the resulting variability observed in the post-discharge narcotic consumption. We excluded six total patients in our analysis who did not complete the pain medication diary as instructed.
Most noteworthy, non-inferiority margins for this study were likely too conservative, particularly in the first 24 hours after surgery where pain management orders allowed for up to 20mg oxycodone every three hours. Our upper confidence limit missed the non-inferiority margin at 24 hours postoperatively by 2.6 MME (e.g., less than 2 mg of oxycodone). We hypothesize that reducing the order to 10mg or 15mg oxycodone for inpatient pain management, consistent with our current protocol, may have established non-inferiority of PAI compared to PNB. Furthermore, reduction in opioid administration protocols may have brought our MME consumption in the first 24 hours more in line with Jarrell et al. (2018), who reported much less opioid use on postoperative day one, in both single- and dual-catheter PNB groups. However, the study groups were not comparable. Jarrell et al. enrolled nearly all types of foot and ankle procedures involving more than the popliteal nerve distribution (Jarrell et al. 2018), while our study examines pain control in TAA, in which the majority of patients had additional procedures (e.g., Achilles lengthening, gastrocnemius release, lateral ligament reconstruction).
There are additional limitations in our approach. First, two surgical approaches (lateral and anterior) were included. We elected to include both systems as this represents the variety of approaches that may be seen within the community. Second, we included all patients who underwent a TAA, regardless of concomitant procedures, which potentially could elicit additional pain. However, there were no significant differences between treatment groups in regard to the proportion of patients who had a lateral versus anterior approach or underwent additional procedures. The treatment groups did differ in their pre-surgical depression screen and FAAM Subjective Ankle Function Scores in that the PNB group self-reported significantly higher depressive symptoms and lower ankle function. Patients with depression are more likely to report increased postoperative pain after joint arthroplasty (Bistolfi et al. 2017; Götz et al. 2021), which may help explain the trend toward decreased mean days to discontinuation of narcotics among the PAI group (19.1 days, sd = 23.6) compared to PNB (25.6 days, sd = 16.1, p = 0.155). Future studies evaluating pain and narcotic consumption following TAA should consider capturing a measure of pain catastrophizing as a potential confounder to postoperative pain assessments (Wood, Gazendam, and Kabali 2021).
CONCLUSION
In conclusion, this study is the first prospective, randomized trial to evaluate postoperative pain and narcotic consumption in a periarticular block compared to a peripheral nerve block. While results did not confirm non-inferiority across all measures at 24 hours and two weeks, there were no significant difference in mean VAS, opioid consumption, or complications identified between study groups. Conservative non-inferiority margins for immediate pain control in the first 24 hours were breached by approximately 2mm on the VAS and 2.6 MME, respectively. With the potential risks associated with PNB, PAI offers a simple, inexpensive and alternative approach to pain control in TAA that should be considered in place of PNB. Since we observed no significant differences in mean opioid consumption or pain scores postoperatively, further research with a greater sample size will likely demonstrate that PAI is no worse than PNB for postoperative pain control and avoids neuropathic complications associated with PNB.



_compared_to_periarticular_injection_(n_23)_.jpg)



_compared_to_periarticular_injection_(n_23)_.jpg)