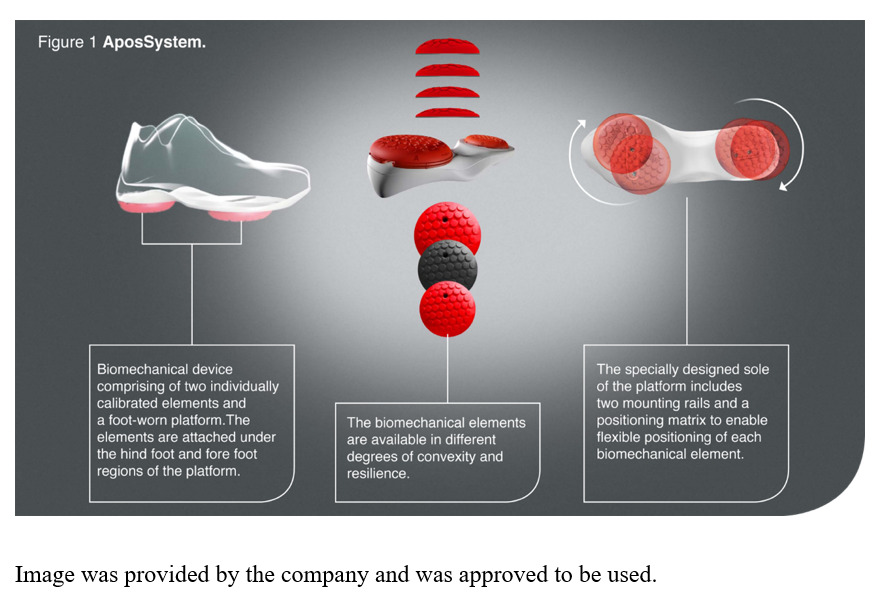
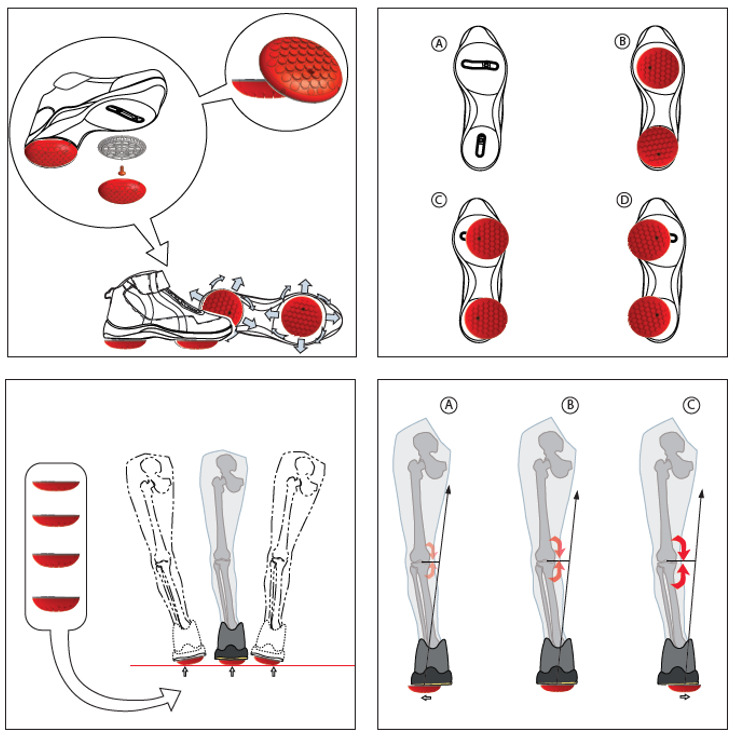
Introduction
Osteoarthritis (OA) is the second most frequent chronic musculoskeletal (MSK) condition after low back pain and a leading cause of disability in the elderly (Storheim and Zwart 2014; Endstrasser et al. 2020). The incidence of knee OA is rapidly increasing due to global demographical changes, mainly an aging population from increased life expectancy and the growing prevalence of obesity. It is estimated that by 2050, 130 million people will suffer from OA worldwide, of whom the disease will severely disable 40 million (Wittenauer, Smith, and Aden 2013), leading to a gradual increase in burden for society.
Total knee replacement (TKR) is a common solution for patients with end-stage knee OA. Over the years there has been an increase in the demand for TKRs attributed to an increase in life expectancies and a decline in the average age of surgical candidates. More recently, an inactive lifestyle exacerbated with COVID-19 can potentially put more patients at risk for TKR (Endstrasser et al. 2020). For the above reasons, the burden of knee OA on society is on the rise (Klug et al. 2020). Existing pharmacological and non-pharmacological interventions for OA remain insufficient. These include physical therapy, biomechanical interventions, oral medications, and injections (McAlindon et al. 2014). There is an urgent need for new non-invasive interventions to effectively treat knee OA and serve as an alternative to surgical intervention.
In this review we will focus on the biomechanical aspects of knee OA and the use of biomechanical interventions to treat knee OA. In addition, we will summarize the scientific evidence of a non-invasive biomechanical intervention that aims to reduce pain and improve function by shifting loads and training neuromuscular control.
Biomechanics of knee OA and biomechanical interventions
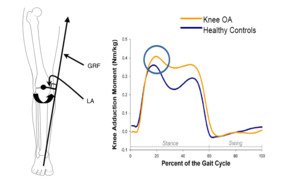
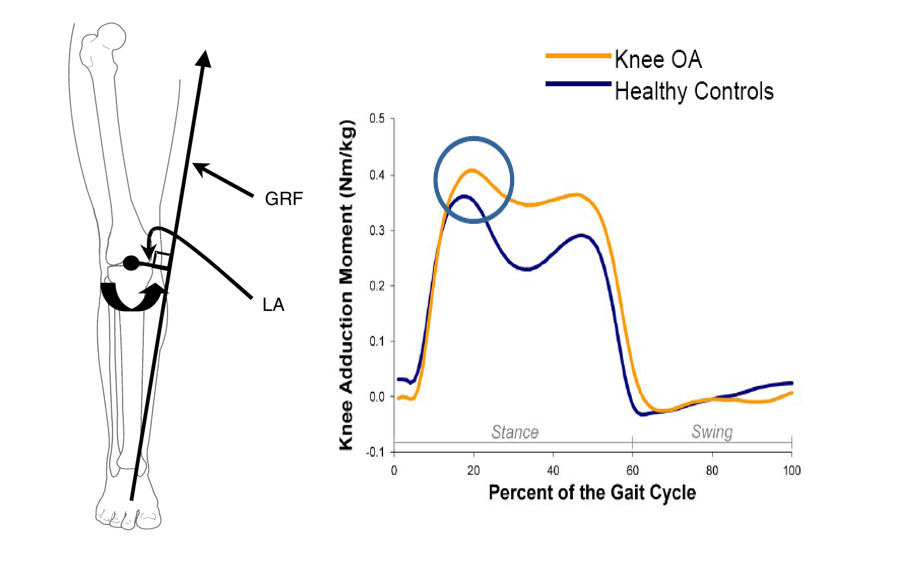
Biomechanics plays an essential role in knee OA. Understanding knee biomechanics is a prerequisite for designing biomechanical knee assistive devices and optimizing rehabilitation exercises for patients with knee OA (Egloff, Hügle, and Valderrabano 2012; Zhang et al. 2020). A typical healthy knee is exposed to 3-dimensional loads (Figure 1). These include the knee adduction moment (KAM), the knee flexion moment (KFM), and the knee external rotation moment (KERM) (Al-Zahrani and Bakheit 2002; Kaufman et al. 2001; Mündermann, Dyrby, and Andriacchi 2005; Gök, Ergin, and Yavuzer 2002). Knee OA is a degenerative “wear-and-tear” disease that occurs most often in people ≥ 50 years of age and affects the biomechanics of the knee joint. The KAM, a primary biomechanical indicator for knee OA, is commonly used to assess disease severity, progression, prognosis and even predict the likelihood of developing future chronic pain in an asymptomatic population. It also correlates with early signs of knee OA, joint space narrowing, medial joint capsule loosening, and symptoms (i.e., pain and functional disability) (Sharma et al. 1998; Amin et al. 2004; Teichtahl et al. 2006; Hurwitz et al. 2002). Wear-and-tear processes also occur to the dynamic stabilizers of the knee, expressed by a deterioration of the neuromuscular control. Patients with knee OA have deteriorated muscle function, including decreased muscle strength and compromised synergy (Messier et al. 1992; Lewek, Rudolph, and Snyder-Mackler 2004). The biomechanical changes justify the use of biomechanical interventions in patients with knee OA.
Biomechanical interventions and walking aids are an integral part of the knee OA care-pathway. Amongst them are footwear, wedge insoles, orthotics, and braces. Some have been included in disease management guidelines (OARSI, ACR, NICE, AAOS) (Carlson et al. 2018; Conaghan, Dickson, and Grant 2008; Richmond et al. 2010; Schnitzer 2002), mainly because they are conservative interventions with low risk. However, in 2021, The American Academy of Orthopaedic Surgeons (AAOS) refined its recommendations on biomechanical interventions and had strongly advised against the use of lateral wedge insoles and advised with a moderate level of confidence on the use of canes and braces to alleviate pain and improve function and quality of life among patients with knee OA (American Academy of Orthopaedic Surgeons 2021).
Although footwear modification has not been officially reviewed and included in knee OA guidelines, there is some evidence on their ability to decrease peak external KAM and reduce pain. These include rocker-sole shoes, flexible/stiffness shoes, mobility shoes, lateral wedge insoles, conventional shoes with changing heel height, and walking with a toe-out (i.e., external rotation) gait pattern. None of the above have sufficient effect on KAM and symptoms (Radzimski, Mündermann, and Sole 2012).
The biomechanical interventions that are endorsed by the international guidelines committee are walking cane and valgus knee braces. Using a cane or a walking stick in the hand contralateral to the symptomatic knee can potentially reduce the peak KAM by 10% (Kemp et al. 2008). However, this might be a limitation when a patient suffers from a bilateral condition since holding the cane in the ipsilateral limb might cause an increase in KAM (Kemp et al. 2008). Valgus knee braces are designed to redistribute the loading in the knee by applying a valgus moment to generate an abduction moment to reduce the KAM and ultimately alleviate knee pain, yet its clinical effectiveness is inconclusive. One of the main limitations of knee braces is compliance. They are bulky, potentially uncomfortable, and might not be a practical daily solution for many patients.
Biomechanical Alteration of Gait (BMAG)
A home-based biomechanical intervention (AposHealth®, New York, US) that includes a unique foot-worn device to manipulate the center of pressure (COP) and train neuromuscular control and a home-based treatment plan has been in use for a few years. The therapy addresses the underlying biomechanical aspects of knee OA (Haim et al. 2008; Haim, Rozen, and Wolf 2010; Haim et al. 2011; Khoury et al. 2013, 2015; Solomonow-Avnon et al. 2015; Solomonow-Avnon, Herman, and Wolf 2019; Solomonow-Avnon et al. 2019; Khoury-Mireb et al. 2019; Debbi, Wolf, and Haim 2012; Goryachev, Debbi, Haim, and Wolf 2011; Goryachev, Debbi, Haim, Rozen, et al. 2011) (i.e., reducing loads by shifting the center of pressure and neuromuscular training), while reducing pain and improving function (Goryachev, Debbi, Haim, Rozen, et al. 2011; Debbi et al. 2015; Haim et al. 2012; Bar-Ziv et al. 2010, 2013; Drexler et al. 2012; Lador et al. 2013; Elbaz et al. 2010, 2011; Elbaz, Mor, et al. 2014; Lubovsky et al. 2015; Herman et al. 2018; Reichenbach et al. 2020; Miles and Greene 2020; Barzilay et al. 2015; Elbaz et al. 2009; Lee et al. 2018; Elbaz et al. 2013; Haim et al. 2013; Atoun et al. 2016; Elbaz, Debbi, et al. 2014; Yaari et al. 2015; Debbi et al. 2019; Drexler et al. 2013; Solomonow-Avnon et al. 2017; Segal et al. 2013; Tenenbaum et al. 2017) (Figure 2). Patients are instructed to wear a personally calibrated device for 30-60 minutes a day while performing their daily activities at home or work (usage time may increase gradually, depending on progress and symptoms). The application of the treatment comprises the functional rehabilitation principle, which stresses the importance of task-specific rehabilitation with repetitive and sub-conscious activities (Levin, Weiss, and Keshner 2015; Charlton et al. 2021). The treatment has a detailed methodology. A trained clinician conducts an in-depth assessment of the patient’s movement patterns and the root causes of their pain. This consultation includes questionnaires related to pain, joint function, and quality of life, an interview, computerized gait analysis, and physical examination. Once the patient has been evaluated, the clinician personalizes the Apos foot-worn device by calibrating the under-sole pods to the patient’s specific needs, validates the location of the pods using subjective and objective measures, and then prescribes a personalized program for the patient (supplement video 1). For example, in medial compartment knee OA, shifting the biomechanical elements laterally causes a lateral shift of the COP that leads to a reduction in KAM (Haim et al. 2008, 2011). Furthermore, shifting the biomechanical elements anteriorly causes an anterior shift of the COP and reduces knee flexion moment (Haim, Rozen, and Wolf 2010).
Clinically, there is growing evidence on the effectiveness of this therapy in several MSK conditions, including knee OA, (Goryachev, Debbi, Haim, Rozen, et al. 2011; Debbi et al. 2015; Haim et al. 2012; Bar-Ziv et al. 2010, 2013; Drexler et al. 2012; Lador et al. 2013; Elbaz et al. 2010, 2011; Elbaz, Mor, et al. 2014; Lubovsky et al. 2015; Herman et al. 2018; Reichenbach et al. 2020; Miles and Greene 2020) low back pain (Barzilay et al. 2015; Elbaz et al. 2009; Lee et al. 2018), degenerative meniscal tear (Elbaz et al. 2013) anterior knee pain (Haim et al. 2013) spontaneous osteonecrosis of the knee (Atoun et al. 2016), total knee arthroplasty (Elbaz, Debbi, et al. 2014; Yaari et al. 2015; Debbi et al. 2019), hip OA, (Drexler et al. 2013; Solomonow-Avnon et al. 2017), total hip arthroplasty (Segal et al. 2013), and recurrent ankle sprain (Tenenbaum et al. 2017). In summary, patients report a significant reduction in pain and improved function and quality of life. In addition, a significant improvement is also seen in objective gait metrics, including spatiotemporal, kinetic, and kinematic parameters. Lastly, there are no serious adverse events related to the treatment, and patients report high compliance with the treatment program (Elbaz et al. 2013).
We classified the evidence into two main areas: prospective clinical trials, RCT, or single cohort 3D motion analysis, done in a controlled environment with a pre-defined, relatively homogeneous patient population. The second one, equally important, is real-life evidence demonstrating the effectiveness in a heterogenic population suffering from multiple MSK conditions, frequently with severe comorbidities. Both methodologies complement each other and address different aspects, yet the effectiveness of the treatment on patients’ symptoms was significant in both routes. Whether in a controlled environment or in real-life clinical practice, the clinical outcomes following treatment meet the gold-standard clinical significance threshold (Copay et al. 2018; Pham et al. 2004).
With respect to knee OA, studies show an improvement in biomechanical parameters and indicators of knee OA while walking with and without the device including a reduction in KAM (Haim et al. 2012), a reduction in knee flexion moment (Debbi et al. 2015), improvement in muscle activation (Goryachev, Debbi, Haim, Rozen, et al. 2011) and improvement in spatiotemporal gait patterns (Lador et al. 2013; Elbaz et al. 2010; Elbaz, Mor, et al. 2014; Lubovsky et al. 2015; Herman et al. 2018). The improvement in biomechanical indicators was associated with improved patient-reported outcome measures (PROMS), i.e., pain, functional disability, and quality of life (Bar-Ziv et al. 2010, 2013; Reichenbach et al. 2020). Recently, a double-blind RCT was published in The Journal of the American Medical Association (JAMA) (Reichenbach et al. 2020). Two hundred twenty (n=220) patients with knee OA were enrolled in a double-blind RCT that compared AposHealth to a sham device. Patients were assigned to one of two groups and were treated for six months. The primary outcome measure was the changes in pain and the secondary outcomes were function, QoL, gait patterns, and adverse events. A significant reduction in pain and improvement in function and quality of life was seen in the BMAG group with an average of 69% reduction of pain. 92% of the patients in the intervention group reported more than 30% reduction in pain, well above the minimal clinical important difference, and 83% of the reported more than 50% reduction in pain, a strong indication of the high efficacy with the number needed to treat (NNT) equal to three (Reichenbach et al. 2020). Another study evaluated the changes in KAM and symptoms of pain and functional disability in a sub-group analysis of disease severity measures by Kellgren and Lawrence (KL 2, KL 3-4) and found both groups to improve significantly. A trend towards increased improvement was seen in the more severe group (Haim et al. 2012). The treatment also seems to have a similar effect on sub-group analysis of age, BMI, and gender (Drexler et al. 2012; Lubovsky et al. 2015).
With respect to long-term data, a two-year follow-up study of patients with knee OA reported maintenance of clinical efficacy seen after 8 weeks over a 2-yrs timespan (Bar-Ziv et al. 2010, 2013). Patients reported a 62% reduction in pain and a 61% improved function with a significant time-by-treatment interaction. Another retrospective study evaluated pain, function, and gait patterns at 12 months and reported a significant increase of 16% in gait velocity alongside a significant reduction of 46% in pain and 45% in functional disability (Lubovsky et al. 2015). Interestingly, BMAG was shown to have a superiority effect as a rehabilitation regimen for patients post-TKR compared to traditional PT – an important fact given the statistics that suggest that 20%-30% of the post-TKR patients are with consistent pain (Yaari et al. 2015; Debbi et al. 2019; Wylde et al. 2011, 2018).
Although there is no published data on the cost-effectiveness of BMAG in knee OA population, one study showed a significant reduction of 58% in rescue medicine during a 2-month trial comparing the therapy to controls (Bar-Ziv et al. 2010). In a different study, the researchers reported that only 3% of patients with degenerative meniscal tear progressed to knee arthroscopy (Elbaz et al. 2013). One double-blind study with long-term two-year follow-up data on decay for total knee replacement reported that 2.6% of patients treated with BMAG required a TKR compared to 31% of patients in the control group, an absolute risk reduction of 28.4% (relative risk reduction of 92%), NNT = 3.5 (Bar-Ziv et al. 2013). There is a need for additional studies evaluating the cost-effectiveness of this intervention as well as the long-term (>2 yrs.) effect.
Some limitations should be acknowledged. First, this article was not aimed to perform a systematic review of non-invasive biomechanical interventions for patients with knee OA. For that reason, we did not conduct a literature review and some information might be missing. Secondly, we provided a summary of evidence of a non-invasive biomechanical intervention and relied on the available scientific evidence to date of the review. There are only two large RCTs that assessed the clinical effect of the treatment and some small-size trials looking at long-term outcomes compared to controls. Although it appears that this intervention has positive results with minimal risks, more trials are warranted to determine the long-term effect of the treatment.
Conclusions
The increased prevalence of knee OA and its associated burden on the healthcare system, society, the individual, and caregivers is worrying. Moreover, the projections of annual rates of TKRs, the end-stage solution for patients with knee OA, and revision TKR are alarming. There is an urgent need for a systematic change to control these projections. In the presence of Value-Based Payment considerations to improve efficiency and effectiveness in delivering medical care, the entire healthcare system should be accountable for both quality and cost of care. With the recommendation of improving population and policymaker awareness of the importance and benefits of managing knee OA, including new solutions for an increasing number of people living with disability associated with knee OA is warranted. The reviewed non-invasive, home-based biomechanical intervention was found to be safe and effective for patients with knee OA and we believe that it has the potential to be of value to patients.