Treatment Goals
The primary goal of knee OA treatments is to reduce pain and maintain function. This goal is the expectation of most, if not all, patients. However, mortality is an issue most patients (and surgeons) do not consider to be a relevant outcome. Nüesch et al. (2011) reported that patients with hip and/or knee osteoarthritis had an increased all-cause mortality rate (standardized mortality ratio 1.55, 95% CI 1.41-1.70). Kendzerska et al. (2017) clarified that the increased mortality was due to cardiovascular (CV) risk. “In a large population cohort, a greater burden of hip/knee OA was associated with higher CV risk; the relationship was explained by OA-related difficulty walking.” Therefore, mobility, and indirectly CV risk, is another important treatment goal. Mobility can be measured with phone apps or pedometers. Lee et al. (2019) noted a reduction in all-cause mortality with increasing step counts in older women. The effect leveled off at 7500 steps/day. Saint-Maurice et al. (2020) found similar results in US adults and found no significant association between step intensity and mortality. Therefore, mobility and/or step counts may be important criteria for considering total knee arthroplasty when patients meet radiographic criteria for surgery.
Natural History of Knee Osteoarthritis
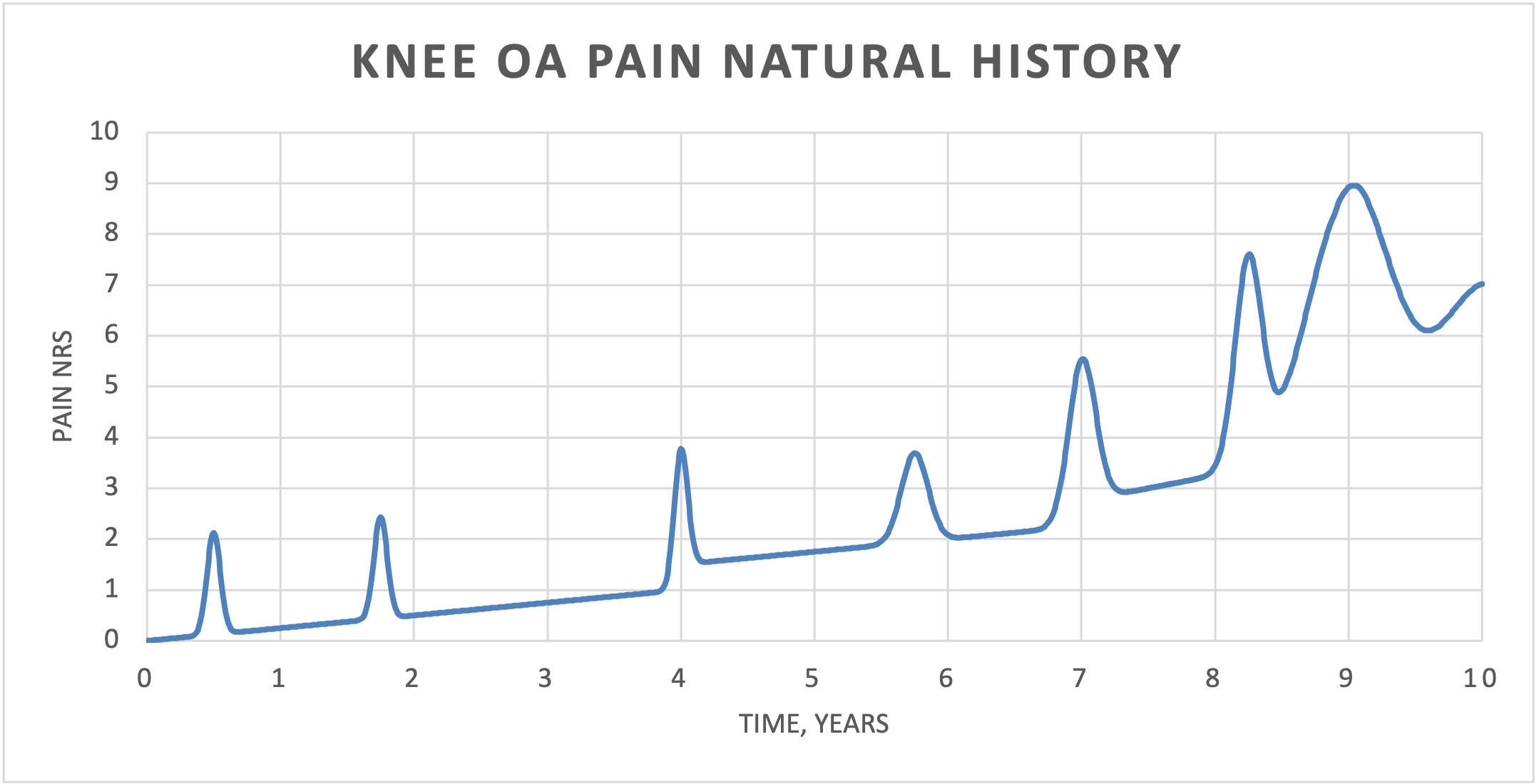
Knee OA is the progressive degeneration of knee articular cartilage. Mild knee OA usually presents with acute episodes of knee pain that are self-limited and improve with time. Because the episodes often improve with time (and without treatment), any treatment initiated during an acute episode will be credited with reducing the pain even though it was simply the passage of time. With time, pain returns to the baseline (mean) pain level (Figure 1). This phenomenon is known as “regression to the mean.” This phenomenon is the reason that randomized controlled trials (RCTs) include control groups to measure the effect of no treatment (time) and/or placebo treatment. As knee OA progresses, the baseline pain gradually increases and the acute episodes become more frequent and more painful. With severe knee OA, the pain becomes more severe and continuous and is no longer relieved, even with effective treatments (Figure 1).
Diagnosis
Patients with knee OA present with “knee pain.” Initial radiographs of patients should include four radiographs:
-
bilateral weight-bearing anteroposterior (AP) view
-
bilateral weight-bearing posteroanterior (PA) notch view (Rosenberg view)
-
lateral view
-
patellofemoral (sunrise) view.
Obtaining weight-bearing views are critical because OA grading systems include cartilage space narrowing as the principal measure of severity. Non-weight bearing views may show “normal” space between femoral condyle and tibial plateau even with severe knee OA. The Rosenberg view is more sensitive at detecting severe OA than the AP view (Eckersley, Faulkner, and Al-Dadah 2021). Kellgren & Lawrence (KL) grading of osteoarthritis is the most commonly used grading system. Typical radiographic features of OA include cartilage space narrowing, marginal osteophytes, bone sclerosis, and subchondral cysts. Because joint space narrowing is the easiest radiographic feature to measure, this author proposes a modified KL grading system (Table 1). The unaffected compartment in the ipsilateral knee or the contralateral knee cartilages spaces can be used to determine “normal” cartilage spaces.
If the knee radiographs show evidence of joint space narrowing (knee OA), a knee MRI is rarely indicated. The exception to this approach would be the patient with mild knee OA radiographically and significant mechanical symptoms consistent with a meniscal tear. In that setting, the patient is being treated primarily for a meniscal tear and the approach to the treatment of knee OA is not appropriate. MRI scans can create patient expectations, such as treatment of degenerative meniscal tears. Because unmet expectations can cause patient dissatisfaction, MRI scans are not recommended if knee radiographs identify knee OA. Identification of degenerative meniscus tears shifts the treatment focus away from knee OA and creates confusion and consternation.
Treatments
The evidence-based treatment recommendations for knee OA are reported in the American Academy of Orthopaedic Surgeons (AAOS) Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline, 2nd Edition (OAK2) (Jevsevar et al. 2013). The three knee OA treatments supported by strong or moderate evidence are: (1) strengthening, self-management programs, low-impact aerobic exercises, and neuromuscular education (Recommendation 1), (2) weight loss for patients with BMI > 25 (Recommendation 2), and (3) non-steroidal anti-inflammatory drugs (Recommendation 7a). Effective knee OA treatments can be remembered with the mnemonic: ANEW.
-
Activity modification – avoid aggravating activities (Strength of Recommendation: Not Applicable)
-
NSAIDs – non-steroidal anti-inflammatory drugs (Strength of Recommendation: Strong)
-
Exercise – self-management and strengthening programs (Strength of Recommendation: Strong)
-
Weight management – recommend weight loss for patients with BMI > 25 (Strength of Recommendation: Moderate)
Activity Modification
Activity modification is self-evident. High impact activities increase knee joint loads. Running, jumping, and high-impact aerobics increase joint loads and knee pain. Changing activities to cycling, swimming, or walking can maintain cardiovascular fitness without aggravating knee OA.
NSAIDs
NSAIDs are the most effective treatment for knee OA. The OAK2 clinical practice guideline (CPG) provides a strong recommendation for all NSAIDS and does not delineate individual oral or topical NSAIDs. A network meta-analysis (NMA) comparing individual NSAIDs, intra-articular (IA) corticosteroids, platelet-rich plasma (PRP), hyaluronic acid (HA), acetaminophen, and IA/oral placebos showed that naproxen is “the conservative treatment of choice” for patients with symptomatic knee OA because naproxen is most likely to decrease pain and improve function (Jevsevar et al. 2018). Additionally, naproxen has the lowest cardiovascular risk of any NSAID (Varga, Sabzwari, and Vargova 2017) and is more cost-effective than celecoxib, opioids, acetaminophen, and corticosteroid injections in older patients with multiple comorbidities (Katz et al. 2016).
Important contra-indications, including renal disease and gastrointestinal symptoms, must be considered before prescribing NSAIDs. When patients are on chronic anti-coagulation, celecoxib may be a reasonable option for knee OA treatment because of minimal platelet function inhibition. When oral NSAIDs are contra-indicated, topical NSAIDs may be used. Diclofenac gel has been shown to be effective (Rannou, Pelletier, and Martel-Pelletier 2016).
Exercise
Lower extremity strengthening allows patients to compensate for their arthritic joint with stronger muscles. Strengthening counters disuse atrophy which can occur with decreasing activity levels as the arthritis and pain progress. Cycling (bicycle or stationary bicycle) is an excellent form of exercise because the patient is non-weight bearing (seated), cycling is a low impact, and cycling can help maintain joint range of motion. Bike geometry is critical for patients and bike geometry (seat height, seat position, handlebar height) should be optimized for each patient. Recumbent bicycles seem to be better tolerated by patients with low back pain. Cycling can aggravate patellofemoral (PF) symptoms for patients with PF OA. Swimming and/or water aerobics use buoyancy to reduce joint forces and pain from weight-bearing. The same reduction in weight-bearing activity can worsen osteoporosis and female patients have a higher risk of osteoporosis. Referral to physical therapy can assist patients in developing a self-directed home exercise program.
Weight Management
The joint forces for the knee are estimated to be 5 times body weight. Therefore, weight loss can have a considerable impact on the patient’s joint forces and pain. The AAOS CPG recommends weight loss for any patient with a BMI > 25 (Jevsevar et al. 2013). Sustainable weight loss requires behavior change and PCPs can help with weight management. Weight loss is critical for morbidly obese patients (BMI > 40). Many guidelines recommend delaying surgery until the BMI < 40 to reduce the risk of infection (The Bree Collaborative 2017). Discussing weight management before patients are surgical candidates provides patients the opportunity to lose weight earlier and not delay surgery when pain is more severe. Overweight patients (25 < BMI < 30) are not likely to receive significant benefit from weight loss so focusing on other treatment options provides more value to the patient.
Corticosteroid Injections
Although corticosteroid injections have been shown to be the most effective knee OA treatment for short-term pain relief (not function improvement) (Jevsevar et al. 2018), corticosteroid injections have two significant side effects that are concerning (Kompel et al. 2019). In a RCT, McAlindon et al. (2017) have shown that multiple corticosteroid injections increase cartilage volume loss and thereby accelerate the progression of OA. Therefore, repeated corticosteroid injections in patients with KL grade 1-2 knee OA have a relative contraindication. Bedard et al. (2017) have shown that a corticosteroid injection within 6-7 months of total knee arthroplasty (TKA) increases the risk of periprosthetic infection. If the patient has KL grade 4 knee OA and surgery is being considered within the next 6 months, injections are contra-indicated. Therefore, corticosteroid injections should be primarily reserved for patients with KL grade 3 knee OA who already have significant OA progression and are not yet surgical candidates or patients that are not surgical candidates or patients that decline surgery.
Acetaminophen
OAK2 found the evidence for acetaminophen use in knee OA to be “Inconclusive” (Recommendation 7b) (Jevsevar et al. 2013). Acetaminophen has few risks and is tolerated well by most patients. This should be considered in patients that cannot tolerate NSAIDs, particularly the elderly.
Genicular Nerve Radiofrequency Ablation
One of the newest treatments for knee OA is genicular nerve radiofrequency ablation (RFA). A recent systematic review found that RFA is superior to corticosteroid injections, hyaluronic acid injections, diclofenac, acetaminophen (paracetamol), and sham procedures (Chen et al. 2021). No significant adverse events have been reported to date. The most promising finding is the potential duration of pain relief with RFA. Hunter et al. (2020) found significant pain reduction at 18 and 24 months after RFA. It is not known if RFA can be repeated or if there are any complications associated with TKA surgery after RFA. RFA may become the treatment of choice for patients that have failed other non-operative treatments and need to manage their knee pain until the knee OA has progressed to KL grade 4, for patients that need to optimize modifiable risk factors, and for patients that are not surgical candidates. RFA is more effective for medial compartment OA than patellofemoral OA (Burgos et al. 2021).
Knee Arthroscopy and Partial Meniscectomy
OAK2 does not recommend knee arthroscopy for chondroplasty/debridement in patients with knee OA (Recommendation 12) (Jevsevar et al. 2013). This recommendation is supported by other systematic reviews (Brignardello-Petersen et al. 2017) and CPGs (Siemieniuk et al. 2017). However, questions regarding the effectiveness of partial meniscectomy in the presence of mild or moderate knee OA remain. The literature would suggest that the more severe the OA, the less likely arthroscopic meniscectomy will be beneficial. The METEOR trial (Katz et al. 2013) found equivalent outcomes between physical therapy (PT) and arthroscopic partial meniscectomy, but high crossover rates from PT to surgery make the results difficult to interpret. However, even if the results of PT and surgery are equivalent, a 6-8 week course of PT should be tried before considering surgery.
Perhaps a more important question regarding knee arthroscopy is: “Does prior surgery compromise TKA outcomes?” Prior to the introduction of evidence-based medicine, our “best available” evidence was level IV case series. Most, if not nearly all, case series had a negative prognostic factor for outcomes: the number of previous surgeries. Scott et al. (2016) noted that the number of previous surgeries was a predictor of patients’ dissatisfaction for TKA outcomes. Fassihi et al. (2021) noted that knee arthroscopy within 2 years of unicompartmental knee arthroplasty (UKA) increased the risks of conversion from UKA to TKA from 4.9% to 10.4%, odds ratio (OR) = 2.11; p<0.001. In TKAs, Gu et al. (2021) found that in a national insurance database which included 130,128 patients, patients that underwent knee arthroscopy ≤ 15 weeks prior to TKA had an OR of 1.79 (p < 0.001) of revision TKA surgery and between 16-35 weeks had an OR of 1.20 (p < 0.035) of revision surgery. Periprosthetic joint infection (PJI) for patients undergoing knee arthroscopy ≤ 15 weeks prior to TKA had an infection rate of 2.7% (p < 0.001) and knee arthroscopy 16-35 weeks prior to TKA had an infection rate of 1.4% (p = 0.004) compared to a control infection rate of 0.9%. Patients undergoing knee arthroscopy up to 1 year prior to TKA had an increased rate of manipulation under anesthesia (MUA): 0-15 weeks – 5.0% (p < 0.001); 16-35 weeks – 4.8% (p < 0.001), 36-43 weeks – 4.9% (p = 0.003); 44-52 weeks – 4.2% (p = 0.065); control – 3.1%. Quinlan, Werner, and Browne (2021) found that previous soft tissue surgery increased revision surgery rates (1.8% vs 1.4%, OR 1.33, p = 0.006). Therefore, the principle of “first do no harm” would suggest that knee arthroscopy for meniscectomy should be considered when there is a high likelihood of long-term symptomatic improvement.
Unloader Braces
The evidence for “unloader” braces (valgus-directing force brace for medial compartment OA) is inconclusive (Recommendation 4) (Jevsevar et al. 2013). Kirkley et al. (1999) found that patients with medial unloader braces performed better than patients with neoprene sleeves in the six-minute walking test (p = 0.021) and the thirty-second stair-climbing test (p = 0.016). Unloader braces are a reasonable alternative for active individuals that need pain relief for specific weight bearing activities and are not ready for surgery. Research suggests that pain improvement with a medial unloader brace is predictive of pain relief with a high tibial osteotomy (Minzlaff et al. 2015).
High Tibial Osteotomy
High tibial osteotomy (HTO), also known as proximal tibial osteotomy (PTO), is an osteotomy to correct the mechanical malalignment of isolated medial compartment knee OA. Initially performed as a lateral closing wedge osteotomy, medial opening wedge osteotomies are now the preferred procedure (Brown and Amendola 2000; Fowler, Tan, and Brown 2000). HTO is an appropriate option for patients with high-demand work or activities that are likely to lead to early failure/aseptic loosening of TKA/UKA components. HTO is preferred to UKA because mean Oxford Knee Scores (OKS) for TKA after HTO are similar to mean OKS after primary TKA while mean OKS for TKA after UKA are similar to mean OKS after revision TKA in the New Zealand Joint Registry (Pearse et al. 2012). HTOs with more recent operative techniques have 79% survival rate at 10 years after HTO (Primeau et al. 2021).
Opioids
Fifty-eight percent of patients received an opioid prescription in the 12 months preceding surgery and 7.2% were on continuous opioid medication for the 12 months preceding surgery (Jin et al. 2019). In addition to all the risks of chronic opioid use, pre-operative opioid use worsens outcomes for joint replacement surgery. Smith et al. (2017) noted that patients receiving pre-operative opioids prior to knee replacement surgery had 19.6% less pain relief on their WOMAC Pain Scores. One year after surgery, 64% of patients on opioids prior to hip or knee replacement surgery were still on opioids for pain (Zarling et al. 2016). Patients taking opioids prior to total knee replacement have a higher risk of early revision surgery than patients not taking opioids prior to surgery (Ben-Ari, Chansky, and Rozet 2017) and reducing their pre-operative opioid use prior to surgery improves their outcomes (Nguyen, Sing, and Bozic 2016). Patient outcomes are better if patients are never started on opioid pain medication prior to surgery.
Tramadol is a mild opioid and received a “Strong” recommendation in OAK2 (Recommendation 7a) (Jevsevar et al. 2013). Driesman et al. (2019) found that pre-operative tramadol use prior to TKA had less improvement in functional outcomes than opioid naïve patients. Therefore, pre-operative tramadol use should be avoided as other opioids should be avoided.
Hyaluronic Acid Injections
OAK2 does not recommend use of hyaluronic acid (HA) injections (Recommendation 9) (Jevsevar et al. 2013) and the strength of the recommendation is “Strong.” This recommendation is supported by multiple meta-analyses which show that although HA is statistically superior to placebo, the difference does not reach clinically significance (Jevsevar et al. 2015). Rutjes et al. (2012) performed a meta-analysis of HA injections and found no clinically significant treatment effects. Irrespective of whether the HA improvements reach clinical significance or not, the relatively high costs of treatment mean that HA injections are a low value treatment (value = outcome/cost). Providers that support the use of HA injections often cite a systematic review by Bhandari (Johal et al. 2016). However, these results should be viewed with caution as Bhandari lists a financial conflict of interest with Sanofi (manufacturer of Synvisc®) in another publication (Bhandari et al. 2017).
Platelet-Rich Plasma and Cell-Based Therapies
The clinical effectiveness of platelet-rich plasma (PRP) and cell-based therapies (CBTs) for knee OA was addressed at an AAOS/National Institutes of Health (NIH) cosponsored conference (Chu et al. 2019). The clinical effectiveness of PRP or CBT treatments is still unproven. Important concerns highlighted by the conference are the variability between PRP treatments/products and variability between patients’ platelets. Given the lack of insurance payment for PRP or CBTs, these treatments are considered low value treatments.
Decision for Surgery
Approximately 20% of TKA patients are not satisfied with their TKA outcomes (Robertsson et al. 2000; Baker et al. 2007; Bourne et al. 2010). Ghomrawi et al. (2020) examined the “timeliness” of TKA surgery. Data was pooled from two cohort studies and included 3,417 knees with knee OA out of which 1,114 TKAs were performed. Of the 1,114 TKAs, 294 were classified as “premature.” Multiple researchers have reported that patients with more severe radiographic OA grade (Kellgren Lawrence) are more likely to have better TKA outcomes (Jacobs, Christensen, and Karthikeyan 2015; Scott et al. 2016; Dowsey, Spelman, and Choong 2016; Rehman et al. 2020; van de Water et al. 2019). Given these findings, TKA surgery should not be performed until the knees have reached Kellgren Lawrence grade 4 or 5. The arthroscopic Outbridge classification of grade 4 (full thickness cartilage loss) or MRI grading of full-thickness cartilage loss are not equivalent to weight bearing KL grade 4 OA and should not serve as alternatives to weight-bearing Rosenberg views for classification of radiographic KL grading.
Van de Water et al. (2019) also reported that lower pre-operative pain scores had better TKA outcomes. Thus, Van de Water found that radiographic severity and pre-operative self-reported pain are two important predictors of TKA outcomes “Patients with less pain and higher KL grades preoperatively had better function and pain outcomes 1 and 2 years after TKA.” Figure 2 shows pre-operative self-reported pain scores for TKAs (unpublished data). The 75% percentile pain score is 7.7. Therefore, approximately 80% of TKA patients would have a pre-operative pain score of ≤ 8.0 and approximately 20 percent of pre-operative TKA patients self-report a pain score > 8.0 (matching the percentage of patients not satisfied with their TKA). Patients with a self-reported pain score > 8.0 may represent pain “catastrophizing” and are likely to have persistent post-operative knee pain (Lewis et al. 2015).
Combining radiographic severity and pre-operative self-reported pain may provide a simple predictive variable. The Arthritis Pain Index (API) is defined as:
\[\text{API} = \frac{\text{Pain score}}{\text{modified Kellgren Lawrence OA grade}}\]
where the pain score is a 21-point Likert scale from 0-10 (0.0, 0.5, 1.0, 1.5, …, 9.0, 9.5, 10.0) and the modified Kellgren Lawrence grade (0-5) is defined above in Table 1. Eliminating a pain score of 0.0 (patient is pain free) and KL grade 0 (patient has no OA), the lowest API is 0.5/5 = 0.1 and the highest API is 10.0/1 = 10. Then log API ranges from log 0.1 = -1.0 to log 10 = 1.0 (-1.0 – 1.0). Using the above analysis where surgery should not be performed until the patient has reached KL grade 4 and pain should be ≤ 8.0, patients with an API ≤ 8/4 = 2 (log API ≤ log 2 = 0.30) should be appropriate surgical candidates.
The Golden Rule
Dr. Kirschenbaum asks an important question in his editorial – “Where are standardized and validated conservative treatment protocols for knee osteoarthritis?” (Kirschenbaum 2021). Evidence supports the use of ANEW treatments (Activity modification; NSAIDs; Exercise/strengthening; and Weight management) as described above. The ANEW treatments are mutually exclusive and can be initiated simultaneously or in stages. NSAIDs should be started at the lowest possible dose and increased as needed. Dosing may need to be increased during acute flares and then reduced when the flare/pain returns to baseline.
The Golden Rule is “treat others as you would be treated.” A measure of your commitment to a treatment approach, protocol, or algorithm is how you treat yourself and/or family. Figure 3 is a weight-bearing posterior-anterior notch radiograph of this author’s knees. My activities were modified and switched from running to walking. Exercise is primarily walking with an average step count of > 19,000 steps per day (just under 8 miles/day). Naproxen 500 mg tablets are taken approximately every other morning with food. Weight management is a daily battle. When discussing weight loss, I tell my patients “I live in a glass house and am not throwing stones.” An unloader brace was used to manage right medial knee pain during an acute flare last year. We have no cure for knee OA; we only manage symptoms. We strive to provide compassionate care to our patients and maximize their function while minimizing their symptoms. Hopefully, my knees provide me with empathy for my patients.



_view_of_author_s_knees._right_knee_med.png)

_view_of_author_s_knees._right_knee_med.png)