1. Introduction
1.1 Lumbar Spine Fusions
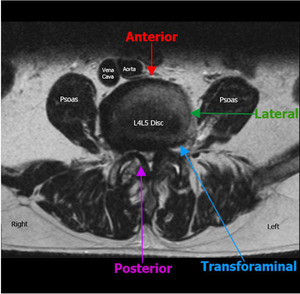
Lumbar spine surgery for the treatment of degenerative pathology has risen substantially over the last several decades, with a trend toward more interbody fusion procedures and minimally invasive techniques (Yoshihara and Yoneoka 2015; Makanji et al. 2018). While a number of different approaches are used for interbody fusion procedures, the lateral retroperitoneal transpsoas approach offers potential advantages over transforaminal and posterior approaches by avoiding retraction of nerve roots, allowing easier access to T12 - L4/5 vertebrae, and not requiring direct access to the spinal canal or neuroforamen (Rihn et al. 2009; Potter et al. 2005; Mobbs et al. 2015). Additionally, the use of this approach permits placement of a larger interbody cage and could potentially promote a higher rate of fusion (Mobbs et al. 2015). Unfortunately, the lateral retroperitoneal transpsoas approach has fallen out of favor due to associated neurologic and vascular complications.
The lateral approach involves creating a “clear path” to the vertebral space by navigating through soft-tissue and (sometimes extensive) dissection of the psoas muscle while being mindful to avoid major neurological structures (Figure 1). To further complicate the matter, locations of both the psoas muscles and lumbar plexus are not uniform and migrate ventrally in the caudal direction. This shift causes a narrowing of the safe operational window surgeons can use to minimize complications in lower lumbar procedures (Regev et al. 2009). Given these circumstances, it is no wonder that a systematic review by Hijii et al. determined that among 63 articles they reviewed with 6819 patients, the top three complications associated with lateral lumbar interbody fusion (LLIF) were transient neurological (36.07%), musculoskeletal (9.22%), and persistent neurological (3.98%) symptoms (Hijji et al. 2017).
1.2 Complications of Lateral Lumbar Interbody Fusion
Several studies have demonstrated that the majority of the noted neurological complications following LLIF are associated with thigh pain, sensory and motor deficits (Hijji et al. 2017; Lykissas et al. 2014). While Lykissas et al. reported that a majority of neurological complications resolved by 18 months, 9.6% of patients had persistent sensory deficits and 3.2% reported persistent motor weakness, which aligns with the findings of Hijji et al. Confounding the issue is the level at which fusion is performed as it has been suggested that the L4-5 level poses a significantly higher risk of femoral nerve injury (Cahill et al. 2012); however, other studies have reported no increased risk (Lykissas et al. 2014).
Musculoskeletal complications are thought to be the result of psoas muscle dissection. This can result in hip pain and hip flexion weakness, but function has been reported as returning to baseline by 2 weeks postoperatively (Knight et al. 2009; Lee et al. 2013; Tohmeh, Rodgers, and Peterson 2011). Hip flexors are not the only musculature affected. A minority of operations have resulted in “pseudohernias,” whereby damage to anterior abdominal motor nerves results in muscle paresis and bulging of the abdomen (Dakwar et al. 2011). Similar to hip flexion weakness, pseudohernias are thought to be transient with no reports of permanent paresis. Lastly, vascular injuries, although more rare, are another complication that should not be minimized. While low rates have been reported (Kueper et al. 2015), vascular injuries are among the most significant postoperative problems associated with LLIF.
One factor that contributes to the rate of postoperative complications after LLIF is the technical difficulty of the procedure. Proper training and a mastery of regional anatomy is crucial to sufficiently avoid vital soft-tissue structures. A substantial learning curve is associated with this approach and was shown to affect the rate of observed complications, with lower rates coming further into a surgeon’s training (Le et al. 2013; Aichmair et al. 2013).
1.3 Intraoperative Imaging
A common factor among the aforementioned postoperative complications is a lack of visualization of key anatomical structures and their subsequent damage. As the popularity of MIS increases and the lateral approach regains traction for interbody fusion, a growing need for intraoperative imaging systems are required to reduce the incidence of neuromuscular complications.
Several devices have been introduced for use in navigation of the lumbar plexus to combat these complications. This includes the use of pre-operative images registered with fluoroscopy, O-arm technology to develop 3D images, and intraoperative MRI (Figure 2). However, these modalities are lacking in their ability to accommodate dynamic anatomy during surgery. An alternative imaging technique involves indirect location of neural structures using directional electromyography (EMG) monitoring (Neuro-Vision, NuVasive, San Diego, CA). This technology is best used for identification of nerves in the path of the lateral approach (specifically around the psoas; Tohmeh, Rodgers, and Peterson 2011; Ebata, Ohba, and Haro 2019).
Currently, directional EMG with neuromonitoring has become the standard for lumbar transpsoas fusions when used with intraoperative neuromonitoring and has been proven to decrease the prevalence of neurological complications (Kwon and Kim 2016; Uribe, Vale, and Dakwar 2010; Rodgers, Gerber, and Patterson 2011; Bergey et al. 2004); however, it is not without its shortcomings. Firstly, the full benefit of EMG is realized only when precise anatomical topography is obtained, and this can rapidly change during surgery (Uribe, Vale, and Dakwar 2010). Additionally, this method is reliant on detection of the femoral nerve as a proxy for localization of sensory nerves and does not allow for the direct detection of afferent fibers (Uribe, Vale, and Dakwar 2010). Furthermore, the ability of EMG to detect nerves is limited by alterations in neuromuscular signal transmission as a result of muscular disease, trauma, or nerve blocking agents (Rabai, Sessions, and Seubert 2016; Holland 1998; Riley et al. 2018). Lastly, it has been proposed that anesthetic and hemodynamic factors as well as problems with positioning or technique may affect its overall accuracy (Riley et al. 2018; Chaudhary et al. 2015; Block et al. 2015).
While EMG may provide some insight regarding the location of key anatomical structures, it still does not meet the need for a real-time intraoperative imaging device. This represents an unmet clinical need for a device that provides surgeons with accurate intraoperative guidance that will help reduce the incidence of postoperative LLIF complications. In this review we will focus on the use of the SonoVision™ imaging system and the Beluga1™ probe (Figure 3) as a potential technology to address the unmet clinical need for real-time intraoperative imaging.
2. Body of Review
2.1 Market Overview
Currently, no effective options for real-time intraoperative soft tissue imaging for spine procedures exist. Commonly used X-ray and fluoroscopic technology do not allow for adequate visualization of soft tissue structures. There are currently several modalities utilized for soft-tissue localization in spine surgery. However, these too present with significant limitations. Preoperative MRIs are registered to images taken throughout the procedure to create a “merged” image, but while such techniques may be somewhat helpful as a reference, it is time consuming and is not always representative of the true anatomical layout, as tissue shifts during surgery. Intraoperative MRI was also at one point suggested as an option, and although it offers high resolution of important neural and muscular tissue, it offers poor differentiation of bone and is unlikely to be used during the procedure (Woodard et al. 2001). Most recently, use of intraoperative CT was shown to provide accurate navigation in LLIF procedures; however, its ability to help identify vital neurological structures is yet to be established (Joseph et al. 2016).
An alternative to imaging is electromyography (EMG), which can provide indirect feedback that may be useful for approximating the location of nerves. Although this technology is currently part of the standard of care for lateral approach spine surgery, it has several important limitations. First, EMG only provides indirect information about the location of nerves and makes the output quite challenging to use effectively. Even with specialized training and carefully focused effort, it allows surgeons to approximate the location of motor nerves only but does not allow for the detection of afferent sensory nerves. Additionally, the methodology of this technique often requires surgeons to disrupt the psoas muscle in multiple locations in order to find a trajectory that avoids vital structures. This can lead to unnecessary damage to the muscle and increase the likelihood of postoperative hip pain or weakness. The accuracy of EMG may be further diminished by several factors including electrical interference, paralytic anesthetics, and several common medical conditions. For an application such as surgical imaging, where inaccurate structure localization can lead to serious iatrogenic nerve injury, this level of accuracy is not sufficient.
Mechanomyography (MMG) is a method similar to EMG that utilizes the mechanical response of muscles to stimuli. One aspect that improves on EMG is a drastic increase in signal to noise ratio and reduction of interference, which collectively improves sensitivity for detection of electrical signals (Bartol and Wybo 2010; Pearlman, Isley, and Ganley 2008; Watakabe et al. 2003). It has been suggested that with the increased ease of signal detection associated with MMG, nerves can be accurately identified without the need for direct visualization (Bartol and Wybo 2010). Although this technology may prove to have increased accuracy over EMG, the ability to avoid and prevent excessive trauma to the psoas muscle and associated nerves using MMG remains unreported and may not be suitable to such procedures as LLIF.
2.2 Introduction to Device
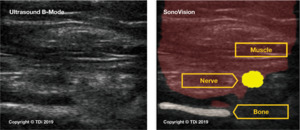
SonoVision™ provides an answer to many of the unmet needs in terms of intraoperative imaging for spine surgery. The use of a machine learning algorithm and numerous rounds of data training allows SonoVision™ to differentiate between various soft-tissue types and structures (bone, nerve, and vascular structures). It uses this information to create a real-time overlay for high-quality ultrasound imaging which highlights and color codes key anatomical structures such as nerves, muscle, bone and blood vessels (Figure 4). While other devices may provide higher image resolution, the tradeoff of near instantaneous images, accurate identification of key soft tissue structures, and low risk and cost for the patient make this a more effective intraoperative imaging device.
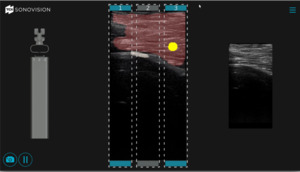
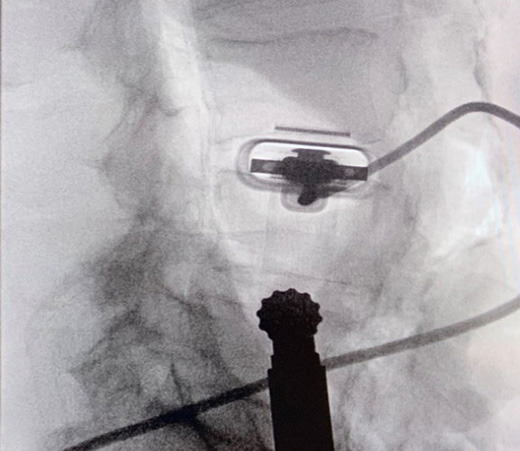
The system utilizes a combination of a tubular stabilizer and ultrasound transducer. Once access to the appropriate disk space is established, the tubular stabilizer is inserted using the same incision site (2.5cm) and positioned over the disk space of interest. The ultrasound is then inserted through the tubular stabilizer and collectively locked into place (Figure 5). Correct positioning is confirmed via fluoroscopy (Figure 6). The real-time ultrasound image with SonoVision™ is then consulted to determine the most appropriate trajectory. The image provides a 25 x 40 mm viewing window and includes overlayed divisions that correspond to 3 potential trajectories available through the SonoVision™ dilator guide. Once a trajectory has been decided upon, the SonoVision™ transducer is replaced with the dilator guide (Figure 7), which features 3 numbered dilator paths corresponding with the 3 potential trajectories displayed on SonoVision™ (Figure 8). At this time, a guide wire can be inserted through the appropriate path in the dilator guide, followed by removal of the dilator guide and stabilizer tube and the use of sequential dilators.
Ultrasound tends to have several distinct advantages as an imaging modality. Among these are its relatively low cost and lack of ionizing radiation. The use of intraoperative ultrasound imaging marginally contributes to the cost of the procedure. The increased efficiency and precision afforded by the SonoVisionTM system is likely to be associated with shorter operative times as well as decreased tissue trauma and risk of neurovascular injury. These improvements will lead to direct cost reductions through more efficient use of operating room time and decreased need for both extended anesthesia and commitment of surgeon and staff time. Additionally, the shorter operative durations and minimization of tissue trauma facilitated by SonoVisionTM mean patients can be discharged and begin their road to recovery earlier and reduce the chances of complications. All these factors may be associated with less direct cost related to the procedure itself, as well as improved secondary costs to the patient in terms of quicker return to work. Conversely more conventional imaging modalities such as MRI, CT or fluoroscopy have their own subset of limitations. MRI and CT can provide a high resolution three dimensional image for intraoperative use; however, this requires not only a time consuming “registration” process but also involves additional imaging during the operation (Ringel et al. 2014; Kim, Bowers, and Chin 2007). The use of rotating c-arms for fluoroscopy does not require a substantial amount of time to complete and eliminates the need for “registration”; however, its efficacy in imaging soft tissue structures is limited and entails substantial radiation exposure.
The other strong advantage of ultrasound technology is the lack of radiation exposure associated with its use. The ionizing radiation associated with other traditional modalities, such as x-ray, fluoroscopy, and CT, may pose substantial long-term risk to patients, surgeons, and operating room staff (Narain et al. 2017). Even with the use of preoperative MRI, multiple intraoperative radiographs may be required for registration, again adding substantial radiation exposure (Tomazevic, Likar, and Pernus 2006). The lack of radiation used by ultrasound translates to decreased risk and relatively unrestricted use with patients. This allows the surgeon to put more focus on the procedure and ensures they have adequate imaging for the full duration.
In preclinical trials, SonoVision’s™ accuracy was assessed based on its ability to correctly and judiciously identify and differentiate tissue types including muscle, bone, nerves, and vasculature. In preclinical animal studies, SonoVision™ demonstrated 100% accuracy for tissue differentiation and identification (Figure 9).
2.3 Clinical Profile
The SonoVision™ ultrasound system is unique among intraoperative imaging devices in its utilization of machine learning to refine the software’s ability to outline and define soft-tissue structures. The scope of this clinical profile will focus primarily on the lengthy and robust process used to obtain training data to optimize the system’s machine-learning algorithm and secondarily on the results of an animal study that aimed to apply and validate SonoVision’s™ trained algorithm and imaging system to an intraoperative setting.
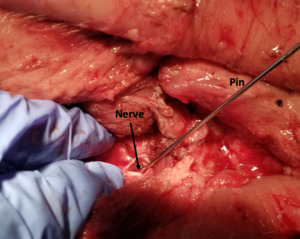
To generate the data sets needed for training of the SonoVision™ algorithm and to determine the device’s ease of use, a combination of benchtop research as well as animal and human studies was employed. First, the capability of the ultrasound system to identify nerves in a variety of different tissues was confirmed. This was accomplished through both an in-vivo rabbit study and a cadaveric study wherein accurate nerve detection by SonoVision™ ultrasound was confirmed by dissection. Once appropriate evidence of the system’s ability to identify nerves was observed, it was prudent to demonstrate that the algorithm driving the software and imaging system was not restricted to simple and controlled scenarios. To this end, an institutional review board approved study confirmed the ability of the SonoVision™ system to locate femoral or ulnar peripheral nerves at various tissue depths in 52 different patients. Following the success of this study, the training dataset was further improved through a subsequent in-vivo porcine study that introduced complex and dynamic tissue environments to assess imaging at multiple disc levels and validate bone targeting functions. At this juncture, testing of the imaging system in a simulated operating room workflow was identified as the next best step.
A simulated usability study applied the trained SonoVision™ ultrasound system with Beluga1TM probe to a surgical setting which was representative of what physicians would encounter in a real scenario. This study involved the use of 5 porcine subjects with all protocols previously approved by IACUC. The primary objective was to assess and validate the SonoVision™ system’s ability to locate nerves, vasculature, and bone and facilitate safe navigation around key soft-tissue structures to access the target disc space. Metal pins represented the “clear-path” to vertebral spaces identified using the system and the presence of nerves or vasculature were subsequently evaluated within the vicinity of each pin. Of the 24 pins placed in the 5 animals, all SonoVision™ imaging notes matched with the corresponding dissection notes representing a 100% accuracy for all tissue type identification (Figure 10).
In addition to imaging of potential nerve locations, the ability of the SonoVision™ software to identify bone in a given image was also tested. Again, pins were inserted through the psoas muscle to the spinal column using the imaging system, and the presence of bone was initially confirmed by the surgeon through touch. All pin locations used for identification of nerve tissue and clear paths were also evaluated for presence of bone and musculature (Figure 11). The Stealth Imaging and Navigation system (Medtronic, USA) was used to confirm the presence of bony surfaces, as well as the depth of both bone and muscle as measured by the SonoVision™ system. Dissection was again used to confirm the presence of target structures. All structures identified and measurements reported by SonoVision™ corresponded with assessments by both surgeon and the Stealth imaging system. This represented accuracy of 100% for identification of bone as well as measurement of tissue depth. Lastly, blood flow imaging was obtained using SonoVision™ ultrasound’s color flow doppler. Blood flow imaging was completed for a total of 24 cases from 3 animals and validated through dissection (Figure 12) and reported accuracy of 100%.
Results from the in-vivo testing were recently submitted along with non-clinical testing for FDA approval. Non-clinical testing of device performance, acoustic output, biocompatibility, cleaning and sterilization, as well as thermal, electrical, electromagnetic, and mechanical safety were all found to be within the recognized standards of the FDA. Based on these results and establishment of comparative use to the predicates Accuro™ 3000 ultrasound system and Esaote 6200 and 6250 Ultrasound System has led to the FDA approval of the SonoVision™ Ultrasound Imaging System with Beluga1™ transducer probe and paves the way for human studies.
2.4 Alternatives and Competition
To date, effective, real-time imaging of soft tissues is not available for spine surgery. This leaves surgeons with an inability to accurately and dynamically visualize vital anatomy prior to and following incision. While options exist to allow for intraoperative imaging of the lumbosacral spine, these methods primarily rely on CT technology and fluoroscopy, which either expose patients and surgical teams to unnecessary amounts of radiation or are cumbersome and time consuming as compared to ultrasound technology (Narain et al. 2017). Therefore, current options for real-time intraoperative imaging in spine surgery are limited to preoperative imaging of soft-tissue structures registered to images collected during surgery (Liu et al. 2018). This is both, again, time consuming and can lead to discrepancies regarding the true anatomical location of vital structures. Without a truly viable option, most surgeons are left with either direct visualization or indirect localization of soft-tissue structures through electromyography (EMG). Although directional EMG is the current standard of care for LLIF, this technology does not permit for visualization of the structures in question and most likely results in multiple passes through the psoas with significant clinical sequelae postoperatively.
2.5 Regulatory Status
SonoVision™ recently received FDA approval for section 510(k), premarket notification of intent to market the device. The intended use is for the visualization and evaluation of nerves, vascular, and other anatomical structures. It is meant to provide assistance in spinal procedure applications.
3. Expert Opinion
Extensive training of the SonoVision™ algorithm involved use of in vivo animal, cadaveric, and human studies, all of which contributed to the accuracy of the device. Subsequently, an in vivo animal trial tested the device’s ability to perform as predicted in a surgical setting. Following this in vivo porcine study, the SonoVision™ ultrasound system proved not only to be user-friendly, but also to provide high sensitivity and specificity for localizing vital anatomical structures that must be avoided during lateral approaches in spinal surgery. Specifically, 100% accuracy was achieved during testing for localization of intramuscular nerves as well as identification and depth measurement of both the psoas and bony surfaces in both the rabbit and porcine model. Given the excellent safety profile of this device, the SonoVision™ ultrasound system positions itself to be in a class of its own when it comes to intraoperative real-time imaging devices for spinal surgery.
SonoVision™ fulfills the currently unmet need for spinal surgeons utilizing the lateral approach. This device allows for true real-time imaging saving both time and tissue intra-operatively. Pre-clinical testing presented evidence that familiarity with the system can be achieved within 15 minutes, making this device even more promising for use in the operating room. Another key advantage of SonoVision™ is reduced dependence on devices that expose patients and OR staff to unnecessary ionizing radiation. The software’s foundation relies on machine learning and the ability to improve both sensitivity and specificity of tissue identification with continued use. This device is poised to become a staple of lateral approach spine surgery in the immediate future and has vast further potential in the broader realm of minimally invasive spine surgery.
4. Five Year Review: Looking Ahead
Devices that enable surgeons to intraoperatively view soft tissue and bone are in demand for a wide variety of applications. Advanced navigation of neuromuscular structures will only add to the trend of improving outcomes for numerous spine procedures. Such a device will address an unmet need in spinal surgery all the while reducing the risk of radiation exposure associated with other imaging modalities.
With ongoing use of the SonoVision™ system, its accuracy in identification and differentiation of tissue structures will continue to progress for a broader array of applications outside of spinal surgery. SonoVision’s™ ability to clearly visualize neurovascular structures will allow for easier and safer administration of nerve blocks for both chronic pain management and peripheral procedures as well as injections for epidural anesthesia. Inadvertent injection of local anesthetic drugs into vascular structures can result in potentially lethal cardiovascular and central nervous system complications and may occur with relative frequency in some applications (Taghavi Zenouz et al. 2008; Hong et al. 2013). Increased ability to localize neurovascular anatomy may reduce the incidence of such complications and increase the precision and effectiveness of anesthetic administration.
Furthermore, real-time visualization and clear differentiation of nerves, blood vessels, and other soft tissue structures via a low-radiation modality may also be highly desirable for vascular and general surgeons. Such technology can increase the safety of complex vascular approaches and reduce uncertainty in high-stakes operative scenarios. In addition, general surgeons and their patients are likely to benefit from increased precision in the administration of local anesthetics and soft-tissue dissection.
All things considered, the possible applications of the SonoVision™ ultrasound system are far reaching and highly exciting. It is capable of substantially advancing surgical practice in the immediate future, and its basis in machine-learning means that its fidelity will only increase with continued use.

_fluoroscopy__b)_computed_tomography__c)_magnetic_r.png)
_sonovision__tm__b)_beluga1_probe.png)



_detection_of_nerve_by_sonovision_ultrasound__b)_confirmation_of_nerve_by_dissection.png)
_detection_of_bony_surface_by_sonovision_ultrasound__b)_c.png)
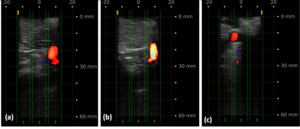

_fluoroscopy__b)_computed_tomography__c)_magnetic_r.png)
_sonovision__tm__b)_beluga1_probe.png)




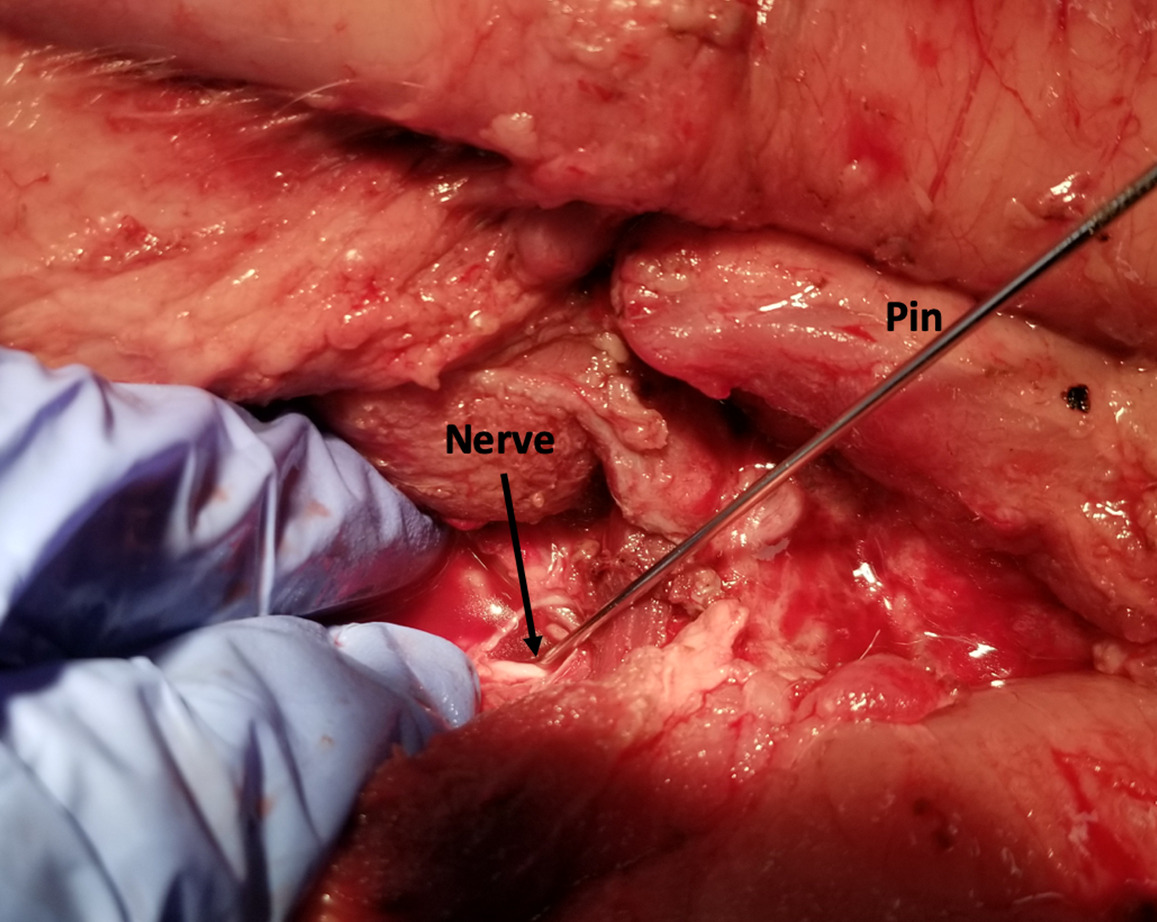
_detection_of_nerve_by_sonovision_ultrasound__b)_confirmation_of_nerve_by_dissection.png)
_detection_of_bony_surface_by_sonovision_ultrasound__b)_c.png)
