Introduction
Shoulder arthroplasty is a fast growing surgical area of orthopaedic surgery (Dillon et al. 2017; Jain et al. 2006). In addition to recent studies showing an increase in the overall rate of shoulder arthroplasty being performed in the United States during the past decade (Kim et al. 2011), the last five years have also seen an explosion of new technology in the field. Patient specific guides derived from preoperative computerized planning software (Iannotti et al. 2019), augmented glenoid components (Ghoraishian et al. 2019), stemless humeral components(Hawi et al. 2016) and virtual reality enhanced technique (Lohre et al. 2020) will likely change the way degenerative conditions about the shoulder are treated in the years and decades to come. It is difficult to predict how fast this new technology will be adopted by the orthopedic community and at what expense.
Looking at the manner in which other technologies have been adopted in prior years could help us to gain insight on what to expect moving forward. The last substantial change in the landscape of shoulder arthroplasty came with the FDA approval of the reverse total shoulder arthroplasty (RTSA) in 2003. The years that followed also demonstrated an increase in the volume of shoulder arthroplasty performed, although to what extent RTSA contributed to the increase in shoulder arthroplasty rates during this time remains unclear (Drake, O’Connor, and Edwards 2010). Previous studies utilizing national inpatient database queries have been able to demonstrate a collective increase in all forms of shoulder arthroplasty procedures being performed by identifying international classification of disease, ninth revision clinical modification (ICD-9-CM) procedure codes for shoulder arthroplasty. However, these studies had been unable to comment on the specific proportion of RTSA because the ICD-9-CM procedure code for total shoulder arthroplasty and RTSA were the same ICD-9-CM procedure code (81.80) until October 2010. This lack of specificity of ICD-9-CM procedure codes has made it difficult to accurately measure the surgical trends of RTSA over time since the FDA approval of the implant in 2003.
The demand for shoulder arthroplasty is projected to increase dramatically in the near future, with growth rates equal to or higher than for hip and knee arthroplasty (Day et al. 2010). An understanding of the changing surgical trends during the years following the adoption of RTSA could potentially help predict what role other new technologies will play in meeting this increasing future shoulder arthroplasty demand moving forward. The aim of this study is to describe yearly surgical trends of RTSA following the FDA approval of this device in the United States, and to review how the rate of RTSA as a percentage of all arthroplasties performed annually has changed over this time.
Materials and Methods
Early surgical trends in RTSA utilization were determined through a two-part investigation of two independent datasets. One database reflected surgical trends on a national level while the second, a registry, reflected regional surgical trends. Inferential statistical analyses were made of surgical trends over time and between national and regional levels.
Part A
For Part A of this study the American Board of Orthopaedic Surgery (ABOS) database was utilized. This database contains all documented cases being performed by candidates taking Part II of their orthopaedic surgery board certification examination. To qualify for Part II candidates must have passed the written examination (Part I) followed by a period of practice of twenty-two months. Candidates then submit reports to the ABOS about all surgical procedures performed during a defined six-month period. Since 1999 the ABOS has maintained a database of these surgical reports, which include procedure dates, primary and secondary diagnosis codes (ICD-9), and procedure codes (Current Procedural Terminology CPT 2009 Professional Edition). Previous studies analyzing surgical trends, such as rates of acromioplasty (Vitale et al. 2010), SLAP repairs (Weber et al. 2012), or treatment of distal radius fractures (Koval et al. 2008), have utilized the ABOS database.
Inclusion criteria for Part A began by searching the ABOS database from 1999-2009 for two CPT codes (23472, 23470). Before October 2010, the CPT code 23472 was inclusive for shoulder arthroplasty, which included total shoulder arthroplasty and reverse total shoulder arthroplasty. CPT code 23470 indicates a shoulder hemiarthroplasty has been performed. To identify all the RTSA cases, every case which met the initial inclusion criteria was reviewed individually for key phrases such as “reverse” or specific component for reverse prosthesis, such as “Delta or glenosphere,” starting in 2005 through 2010. The following cases were also included: “revision RTSA,” “conversion of hemiarthroplasty to RTSA,” and “conversion of TSA to RTSA.” Once these cases were identified, patient demographics including age and gender were obtained and analyzed. From these data collected, the total number of RTSA cases reported was compared to the total number of hemiarthroplasties and total shoulder arthroplasties cases performed in the same time period (2005-2010). In addition, the ABOS database records procedure volume per individual surgeon, and therefore the volumes of all shoulder arthroplasties were reported per candidate. From these data we analyzed the percentage of RTSA cases being performed as compared to the number of hemiarthroplasties and total shoulder arthroplasties performed by each individual candidate per year.
Part B
Part B of the study utilized a regional integrated healthcare system implant registry to identify all forms of shoulder arthroplasty cases performed during the same time interval as Part A (2005-2010). Patient demographics including age and gender were obtained. Each case was then confirmed to be either RTSA, hemiarthroplasty, or total shoulder arthroplasty. The implant registry coded RTSA independently from the onset and did not rely upon ICD-9-CM procedure codes.
Statistical Analysis
An independent biostatistician performed the inferential statistical analysis. Parametric comparisons were conducted with use of unpaired two-tailed t tests for two-sample comparisons. Fisher exact tests were performed for contingency tables and chi square tests were performed for larger contingency tables; Statistical significance for all tests was selected at p<0.05. Regression analysis was performed on the datasets to assess for associations with year as a general trend.
Source of Funding
There was no external source of funding for this study.
Results
Part A
Demographics recorded for ABOS patients receiving shoulder arthroplasty included gender and age. For patients receiving RTSA, 35.3% of patients were male and 64.7% female. The average age was 72.7 years for males and 73.6 for females (SD 9.8 for men, 7.9 for women). For patients receiving TSA, 44.7% were male and 55.3% female, with an average age of 65.2 and 69.2 respectfully (SD 10.7 for men, 10.5 for women). For patients receiving shoulder hemiarthroplasty, 36.5% were male and 63.5% were female, with an average age of 62.2 for males and 69.8 for females (SD 13.3 male, 12.6 female) (Table 1).
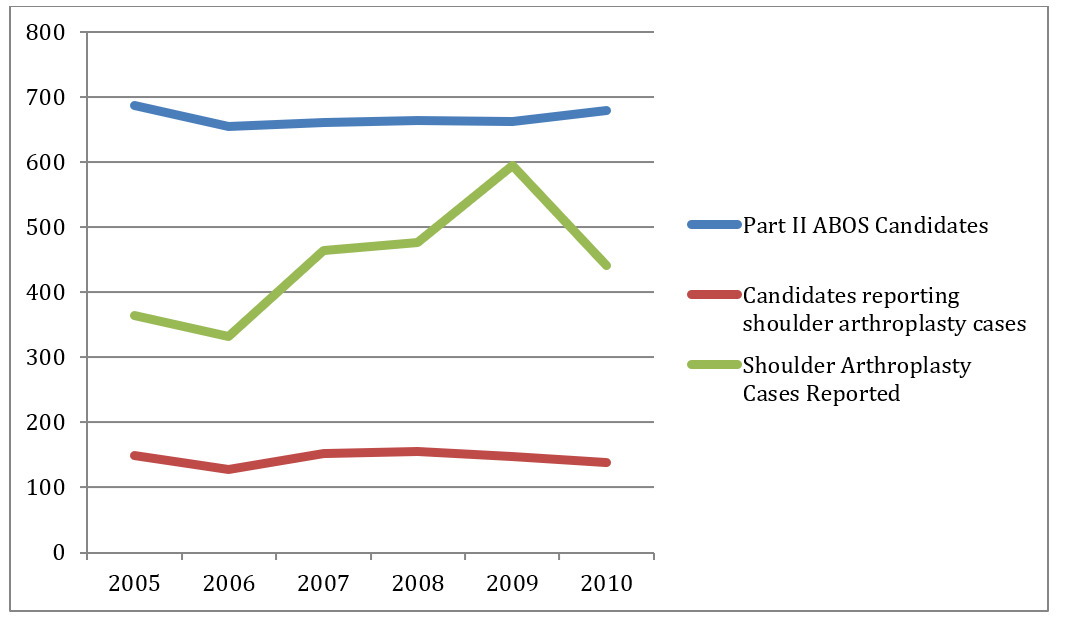
During the period of 2005-2010 the total number of surgeons who submitted cases to ABOS for the purpose of Part II of the orthopaedic surgery boards did not change significantly (Table 2). In 2005, 687 candidates reported cases compared to 680 candidates in 2010. Similarly the number of candidates submitting at least one shoulder arthroplasty (hemiarthroplasty, TSA, and RTSA) case did not change significantly during this time period. In 2005, 149 of the 687 candidates reported at least one shoulder arthroplasty case, while in 2010, 138 of the 680 candidates reported at least one shoulder arthroplasty case. The overall number of shoulder arthroplasty cases submitted each year increased annually during this same time period as well (Figure 1). In 2005, 364 shoulder arthroplasty cases were reported to ABOS. Of these 364 cases, only 19 RTSA cases were reported, or 5.2% of all shoulder arthroplasty cases reported. In comparison, 441 shoulder arthroplasties were reported in 2010, of which 108 were RTSA cases and constituted 24.5% of all shoulder arthroplasty cases reported. Between 2005 and 2010, there was a statistically significant increase in the annual percentage of RTSA reported to the ABOS of 369% (p<0.0001).
The percentage of TSA cases reported also increased during this time period but did not reach statistical significance. In 2005, 131 TSA were performed constituting 36.0% of all shoulder arthroplasty cases performed, while in 2010, 188 TSA were performed constituting 42.6% of reported shoulder arthroplasty cases. Between 2005 and 2010 the percentage of TSA performed increased by 18% (p=0.0599).
During the same time period the percentage of shoulder hemiarthroplasty performed significantly decreased over this time period. In 2005, 214 shoulder hemiarthroplasties were reported constituting 58.8% of cases, while in 2010 just 145 cases were reported constituting 32.9% of shoulder arthroplasty cases reported for an annual decrease of 44% (p<0.0001) (Figure 2).
Part B
Data obtained from the regional integrated healthcare system implant registry showed that during the period 2005-2010 the number of all forms of shoulder arthroplasties performed was 1519, of which 173 were RTSA. Patients receiving RTSA were 58% female and 42% male, with average ages of 75 for females and 74 for males. Patients receiving TSA were 54.8% male and 45.2% female with average age of 71 and 68 years, respectively. Patients receiving shoulder hemiarthroplasty were 50% male with average age of 67 for males and 64 for females (Table 3).
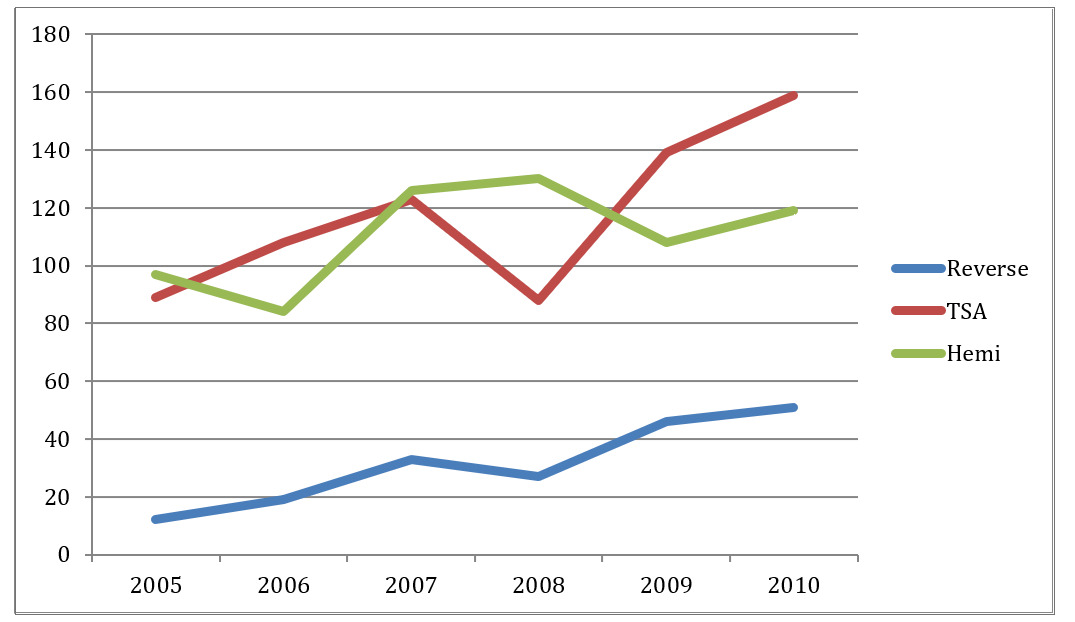
In 2005, just 11 RTSA cases were performed and constituted 5.7% of all shoulder arthroplasty cases performed that year. In 2010, 51 RTSA cases were performed and constituted 15.5% of all types of shoulder arthroplasty cases performed that year (Table 2). The regional increase in RTSA performed was 173% over this time period (p=0.0010) (Table 4).
Concurrently during the same time period, 690 TSA were performed. In 2005, 86 TSA were performed constituting 44.3% of all arthroplasties, compared with 159 cases, or 48.3%, performed in 2010. The annual proportion of TSA compared to all arthroplasties performed increased just 9% over the 6-year period.
The total number of hemiarthroplasty cases performed during this time period was 656 cases. In 2005, 97 shoulder hemiarthroplasties were performed constituting 50.0% of all shoulder arthroplasty cases performed that year compared with 119 cases performed in 2010, or 36.2% of all shoulder arthroplasty cases performed that year. The annual proportion of hemiarthroplasties performed decreased by 18% over the 6-year period (Figure 3).
Discussion
Since the FDA approval of RTSA in November of 2003 (Food and Drug Administration 2003), the proper surgical indications and patient selection for this implant have been repeatedly examined (Cazeneuve and Cristofari 2010; Cuff et al. 2008; Drake, O’Connor, and Edwards 2010; Flatow and Harrison 2011; Martin and Iannotti 2008; Matsen et al. 2007; Sanchez-Sotelo 2009; Vitale et al. 2010; Wall et al. 2007; Werner et al. 2005). Initially, the RTSA was reserved for the low demand, elderly patient with painful pseudoparalysis from rotator cuff arthropathy (Sirveaux et al. 2004; Werner et al. 2005). With these narrow indications in mind, it was initially estimated that RTSA would comprise a small percentage of all shoulder arthroplasty procedures compared with hemiarthroplasty and total shoulder arthroplasty (Cuff et al. 2008; Drake, O’Connor, and Edwards 2010; Wall et al. 2007; Werner et al. 2005). With time, multiple authors have described expanded indications for the use of RTSA procedure, such as acute fractures, the sequelae of trauma, chronic instability, massive rotator cuff tears without arthritis, revision arthroplasty and even recently primary glenohumeral osteoarthritis with a biconcave glenoid (Acevedo et al. 2014; Boileau et al. 2006; Cazeneuve and Cristofari 2010; Cuff et al. 2008; Drake, O’Connor, and Edwards 2010; Gerber et al. 2009; Guery et al. 2006; Martin and Iannotti 2008; Mizuno et al. 2013; Mulieri et al. 2010; Sanchez-Sotelo 2009; Wall et al. 2007).
Our study utilized both a nationwide database as well as a regional integrated healthcare system implant registry to describe overall rates of reverse total shoulder arthroplasty as compared to hemiarthroplasty and total shoulder arthroplasty from 2005-2010, to document the initial acceptance and integration of this option for shoulder arthroplasty surgeons. The ABOS database looks at the first two years in practice for orthopaedic surgeons, whereas the regional integrated healthcare system implant registry looks a data from surgeons in all experience levels, but there is no difference in those two groups.
The ABOS database collects case submission information for candidates applying for Step II of the board certification process. By reviewing this data, we were able to more accurately describe the changing utilization and patient demographics for reverse total shoulder over time on a national level. Our results showed that from the time of FDA approval in 2004 there has been a nonlinear increase in the annual proportion of RTSA cases performed. In 2005 the RTSA rates comprised just 5.2% of all shoulder arthroplasty procedures while RTSA comprised 24.5% of all shoulder arthroplasty procedures in 2010. During the 6-year period (2005-2010) where RTSA saw a 369% increase in use, the overall number of all shoulder arthroplasty cases performed increased but the number of surgeons performing shoulder arthroplasties did not. During the same period, the rates of total shoulder arthroplasty also increased, but less dramatically, while the rates of shoulder hemiarthroplasty decreased. Patient demographics did not change dramatically over time for RTSA, TSA, and hemiarthroplasty cases. A more recent look at the ABOS database until 2017 continued to show increased RTSA use among board eligible orthopaedic surgeons along with an increasing trend of complications (Weber et al. 2021).
A nationwide database of overall procedure rates cannot always accurately reflect regional trends in a surgical procedure. To assess if regional trends tracked national changes, our study also utilized a regional registry to determine rates of shoulder arthroplasty over the same period of 2005-2010. Data obtained from a regional integrated healthcare system implant registry showed that during the period of 2005-2010, the overall annual rate of all forms of shoulder arthroplasty increased, with the relative increase in RTSA most dramatic.
From these data it can be concluded that the markedly increased utilization of RTSA substantially contributed to the overall rise in shoulder arthroplasty rates seen on both the nationwide and regional level from 2005-2010. Interestingly while annual rates of RTSA dramatically increased and TSA rates increased steadily, there was a decrease in the relative proportion of hemiarthroplasty procedures performed, both on a national and regional level.
A study by Kim, et al. used a nationwide inpatient sample to estimate the overall rates in shoulder arthroplasty procedures in the U.S. from 1993-2008 (Kim et al. 2011). Utilizing surgical procedure codes to identify hemiarthroplasty and total shoulder arthroplasty procedures, the study described a steady increase in hemiarthroplasty procedures but a nonlinear accelerated growth in total shoulder arthroplasty CPT code rates, particularly after 2004. As the CPT codes for total shoulder arthroplasty and reverse total shoulder arthroplasty were the same (23472) at the time of their study, the two implants are indistinguishable unless a chart review is performed for each procedure code. The authors speculated, but could not prove, that the increase in shoulder arthroplasty CPT codes after 2004 was due in part to the approval of RTSA.
Schairer et al. reported the nationwide inpatient sample for shoulder arthroplasty rates in the U.S. during 2011, after the separation of ICD-9 procedure codes for RTSA and TSA (Schairer et al. 2015). Their report on 66,485 shoulder arthroplasties performed showed a 33% RTSA, 44% TSA, and 23% hemiarthroplasty rate. Additionally another recent study by Jain and Yamaguchi assessed the utilization of primary shoulder arthroplasty and was able to estimate the contribution of RTSA for just one year of the 3-year time span of the study (Jain and Yamaguchi 2014). The authors concluded that utilization of primary shoulder arthroplasty significantly increased from 2009 to 2011 with a major contribution from RTSA in 2011. The authors could only calculate values for RTSA for one year from a Nationwide Inpatient Sample database from the United States Healthcare Cost and Utilization Project as an independent ICD-9 code for RTSA was not uniformly available until then. The two parts of our study either had validated case reviewed RTSA cases (part A) or the use of RTSA was part of the basic data acquired at the time of the case due to rigor of the registry (part B). Additionally our study assessed changes over a 6-year period in both parts (2005-2010).
Boguski et al. used 2012 supply-chain data in a novel way to determine implant choice in 3,119 shoulder arthroplasty cases from 100 hospitals in 18 states as part of a quality improvement investigation to define shoulder arthroplasty variation (Boguski et al. 2013). The authors reported a wide variation in the use of RTSA across the hospitals queried (0 -100%, mean 42.3%). They also found that hospitals with a high TSA volume had a lower variation in RTSA use than low-volume hospitals. Their study revealed a location-dependent variation of shoulder arthroplasty procedures being performed across hospitals at one point in time. While the supply-chain data is an intriguing mode of analysis, the authors divulge methodological limitations. Specifically, supply-chain data obtained from a very low shoulder arthroplasty volume hospital would likely report an artificially extreme percentage of use of either RTSA or TSA that may alter the statistical analysis of regional variance. They pointed out that in the future, quality improvement collaborative efforts would need to be built around shoulder arthroplasty registries to avoid regional variations in shoulder arthroplasty procedures. The data from our multiyear study in part B comes from a regional registry and can be used to estimate regional variation compared to national rates of shoulder arthroplasty, as was done here.
This retrospective descriptive study limited us from drawing precise conclusions around the reasons why there was an increase in reverse total shoulder arthroplasty. It was possible that RTSA rates were increasing because surgeons had responded to an increased demand within the existing indications of elderly low-demand patients with painful rotator cuff arthropathy and functional shoulder pseudoparalysis. It was also possible that surgeons were expanding surgical indications outside of rotator cuff arthropathy to include treatment of massive rotator cuff tear without arthritis, acute fracture treatment, osteoarthritis with an intact cuff, post traumatic reconstruction, or revision of failed arthroplasty, but we could only speculate from this study data that is presented.
Several studies since have shown an increase in utilization of RTSA in the treatment of proximal humeral fractures that coincides with a decrease in the use of hemiarthroplasty (Dillon et al. 2019; Haasters et al. 2016; Han et al. 2016; Hasty et al. 2017; Jo, Lee, and Lee 2019). Hemiarthroplasty had traditionally been the gold standard for displaced proximal humeral fractures in the elderly. In a study by Hasty et al, the US Medicare population was examined to determine the rates of RTSA and HA use in 750,426 proximal humeral fracture cases from 2005 – 2012 (Hasty et al. 2017). To address the issue of RTSA not having an ICD - 9 code until 2010, the authors described how anatomical TSA is rarely used for proximal humeral fracture as this injury rarely involves the glenoid. From this, they decided to treat the procedures coded as an anatomical TSA as RTSA. The results showed the use of RTSA increased by 406% while the use of HA decreased by 47% during the study period. The authors also found that the use of RTSA, which was originally recommended in patients age 70 years or older, increased by 244% in patients younger than 70 years old during the study period.
In a recent study by Dillon et al, the same regional integrated healthcare system implant registry from part B of our study was utilized to analyze the use of arthroplasty for treatment of proximal humeral fracture (Dillon et al. 2019). Different from the Hasty study, the Dillon study utilized internal codes to differentially identify RTSA from the outset so its data truly represented validated RTSAs. The authors found an increase in RTSA usage with a simultaneous decrease in HA usage during the study period. Furthermore, the utilization of RTSA for proximal humeral fracture surpassed that of HA in 2015 for the first time in this integrated healthcare system.
Another aspect that may be involved in the increased use of RTSA is in the setting of revision procedures. A common complication associated with anatomical TSA is loosening of the glenoid component, which may require revision (Bohsali, Bois, and Wirth 2017; Gonzalez et al. 2011). In another study by Dillon et al, data from the regional integrated healthcare system implant registry used in part B of our study was examined to analyze occurrence of revision following 5,566 anatomic TSAs (Dillon et al. 2020). The data revealed 4.1% of cases underwent revision, 1.4% of which had glenoid loosening as the primary indication. In a study by Wagner et al, 274 revision arthroplasties were examined from 2005 to 2016 (Wagner et al. 2019). The authors found that revision arthroplasties to RTSA increased from 51% in the first half of the study to 78% in the last half compared to the use of anatomical TSA or HA for revision. Furthermore, the frequency of revision to RTSA with glenoid loosening as the indication increased from 17% to 86% during the same time.
Surgical indications were not uniformly reported by all of the ABOS Part II candidate case submissions and therefore any analysis of surgical indications would be incomplete. Additionally, it was unclear why hemiarthroplasty rates slowed or decreased in overall proportion during the time period. It could not be inferred from the study data presented that surgical trends utilizing RTSA had replaced hemiarthroplasty for specific surgical indications or patient demographics but later data did reveal this trend to occur as mentioned above. Furthermore this data does not describe who was performing RTSA and who was responsible for the large increase in case incidence. It cannot be determined if these trends are due to the increased number of surgeons completing shoulder arthroplasty fellowships (Day et al. 2010), or if in fact shoulder fellowship trained surgeons are performing the vast majority of these cases.
The lack of clarity around how the orthopaedic community had initially accepted and utilized the added option of RTSA in the first years after FDA approval drove our inquiry. It has been well established that orthopedic surgeons do not always fully appreciate the costs of the implants utilized at surgery (Arliani et al. 2016; Okike et al. 2014; Streit et al. 2013), leading some to call for the adoption of specific strategies for the implementation of new technology into practice (Hannan et al. 2018; Pijls and Nelissen 2016; Wyatt et al. 2020). To this end, Pijls and Nelissen stressed the importance of post-marketing surveillance in identifying poorly identifying prostheses, specifically pointing to the role that joint registries play in this process (Pijls and Nelissen 2016).
Conclusion
This study describes early surgical trends in shoulder arthroplasty by comparing two existing datasets on the national and regional level. These early results are complemented by existing literature that corroborates that the overall rate of shoulder arthroplasty procedures performed has increased from 2005-2010 and beyond. More specifically, this study described a dramatic, non-linear, increase in annual rates of reverse total shoulder arthroplasty compared to a less dramatic increase in total shoulder arthroplasty and a relative decrease in shoulder hemiarthroplasty rates. The increasing rates of use of RTSA, as well as the increase in RTSA as a relative proportion of all shoulder arthroplasties, was seen both on the nationwide and regional level data. These comparative results of both datasets may reflect a more uniform national increase in RTSA performed and not a regional bias.
The rise of RTSA use has continued since these early documented rates with newer literature supporting increases in indications and volumes. This report serves as a retrospective comparison of rates early on to more recent RTSA use. As prior studies have described complications such as postoperative dislocation, glenosphere dissociation from baseplate, scapular notching, acromial/scapular spine fracture, reoperation and higher morbidity and VTE rates, the increased utilization of RTSA will continue to be monitored along with continued assessment of value as measured by quality and outcomes (Acevedo et al. 2014; Boileau et al. 2006; Cazeneuve and Cristofari 2010; Cuff et al. 2008; Flatow and Harrison 2011; Guery et al. 2006; Jain and Yamaguchi 2014; Martin and Iannotti 2008; Mulieri et al. 2010; Navarro et al. 2013; Wall et al. 2007; Werner et al. 2005).
The use rates early in the experience of the innovative idea of RTSA may be instructive and educational to surgeons in this time of expanding indications for RTSA and with utilization of newer RTSA designs.