Orphans in medicine are unusual, frequently debilitating, or deadly conditions for which there are no or few treatments. The medical industry, primarily pharmaceutical, will develop these necessary treatments and can obtain expedited regulatory approval. This method does not exist in surgical or combination devices, where we live as orthopedic surgeons. We can divide the solutions into instruments or implants. I want to keep this to instruments for now. Instruments are typically a class 1 regulatory device. Sterilization and reuse of the instrument must be clearly defined. I, like most orthopedic surgeons, can be very instrument-centric. A well-designed instrument can make a procedure more reliable and reproducible. We have all experienced poorly designed instruments and have shared experiences in the surgical technique struggle. There are times where there is no instrument, and that adds to operating room angst. I want to propose that an open-source “Wiki” develop surgical “orphan” instruments. These instruments would not be be patent protected and are open to all, similar to surgical procedures.
Recently I was contacted by a young orthopedic surgeon who wanted to start a business based on a jig. This jig has value and does solve a problem; the difficulty is selling jigs alone as a value proposition. Healthcare has many masters to serve: the patient, the surgeon, the hospital system, the payor - commercial or government, the regulatory agencies, the manufacturer, and its shareholders.
The large and small orthopedic companies are not in the business of making “special” tools. There are a significant regulatory burden and potential liability. The instrument companies may make an instrument, but the costs can be pretty high for a “one off.” The costs of patenting instruments with little commercial value must be weighed with the tens of thousands of dollars in costs and up to 5 years in time. The regulatory issues are another problem. Meanwhile, we continue to struggle when one of our colleagues has the solution in their back pocket and no method to disseminate the solution.
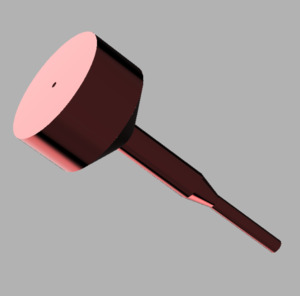
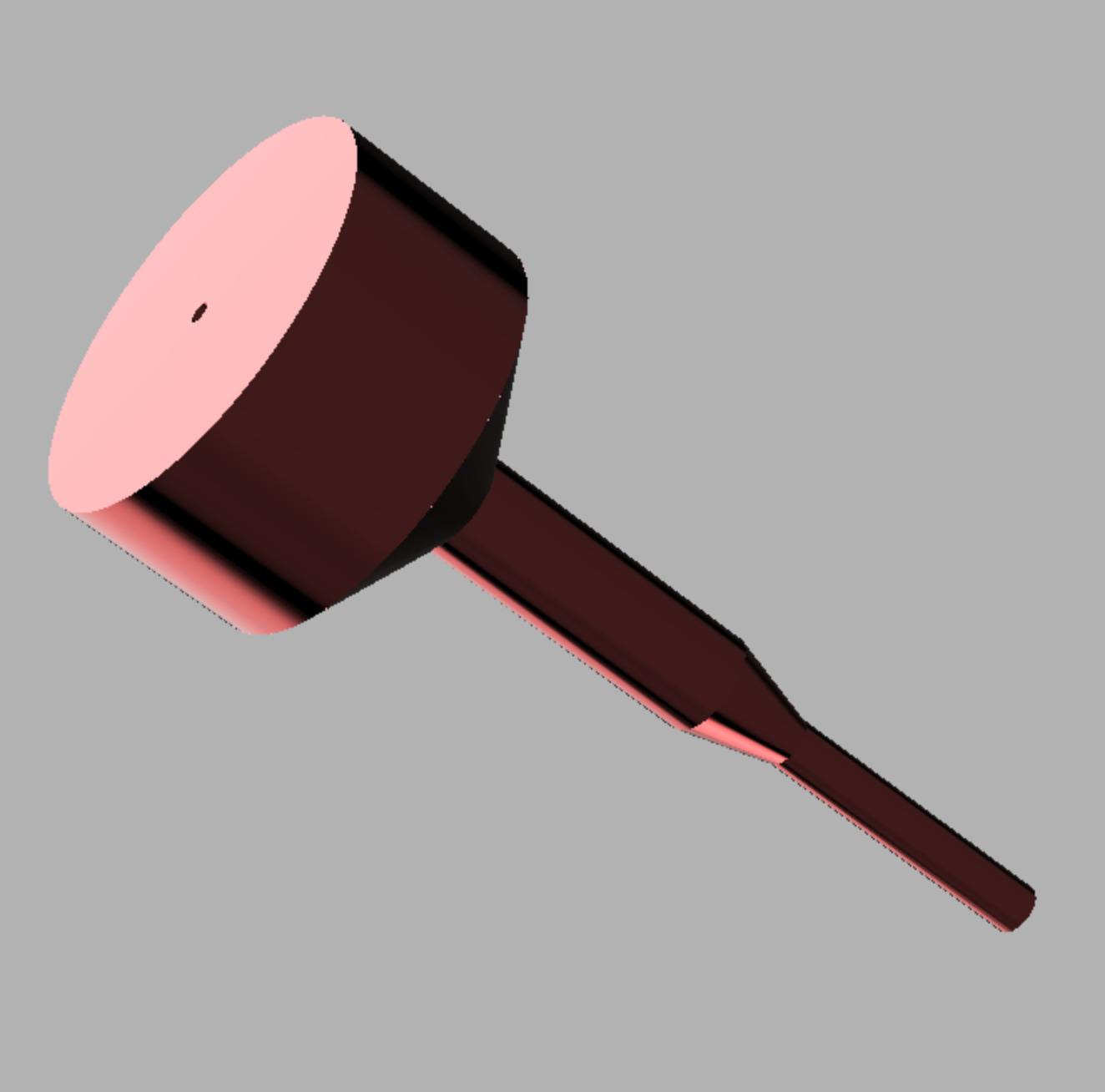
I am presenting the conceptual groundwork for changing the surgical technique paradigm and is just an extension of “community sharing” of surgical techniques by adding instrumentation. I would like those in the industry to comment as well. Would they be open to manufacturing these instruments and working with the orthopedic community to refine these instruments.? Much of the global orthopedic community would greatly benefit from the sharing of these ideas. This cannulated bone tamp concept came to me when I listened to a presentation from an Indian surgeon discussing their technique for ORIF of the calcaneus. I thought that having a guide-wire to provide subchondral support would be facilitated by a cannulated tamp. I would also add to the tamp a right angle guide for the raft screws. This instrument is implant agnostic; the cannula size can be modified based on the guidewire’s needed size. This would allow any cannulated screw from any manufacturer to be selected. This was presented to an instrument manufacturer and was rejected because they believed that this fell under an implant company’s domain. So it sat in my back pocket until now.
There will be a need to learn new skills, primarily CAD and using a 3D printer. If this seasoned orthopedic surgeon can learn these skills, it should be pretty easy for surgeons in this current generation. Prototypes can be made, and the files can be refined quickly by a global community of orthopedic surgeons. Simple modifications or complete rewrites are encouraged. The tamp file was sent to an online manufacturer, fabrication in aluminum would cost 78 USD and take one week from receipt of the digital file.
Consider this opinion the starting point for the discussion; all opinions are welcome, but let us determine the steps forward if there is a consensus. The CAD files can be loaded to www.grabcad.com so anyone can modify this instrument as needed. At this time, there are over 4.6 million CAD files freely available and none in the medical section.