Introduction
The first glenoid component was a cemented all-polyethlene keeled implant introduced by Charles Neer in 1974 (Neer 1974). He later published his results, showing good outcomes with improvements in range of motion and function (Neer, Watson, and Stanton 1982). There have been significant advances and innovations of the glenoid component in total shoulder arthroplasty (TSA) since 1974, enhancing the success of the procedure today. Long-term data supporting its use in the setting of glenohumeral arthritis shows excellent results. Cofield reported revision free survival rates of 94.2%, 90.2%, and 81.4% at five, ten, and 20 years, respectively (Singh, Sperling, and Cofield 2011).
While excellent outcomes are seen in the setting of glenohumeral arthritis and an intact rotator cuff, there are high rates of early failure with concomitant rotator cuff pathology (Franklin et al. 1988). Historically, the only treatment option for these patients was hemiarthroplasty or humeral resurfacing. The poor outcomes in this patient population inspired the design of the reverse shoulder arthroplasty (RSA). Since approved for use in the United States in 2003, the use of RSA has significantly improved clinical outcomes in patients with glenohumeral arthritis and a nonfunctioning rotator cuff. Indications have only increased since.
Outcomes for shoulder arthroplasty are generally favorable, though certain scenarios have been associated with decreased longevity of the implant, inferior results, and increased complications. These situations include glenoid bone loss, revision arthroplasty, and arthrosis in the young, active patient. Recent innovations exist to address these scenarios and have demonstrated early success. Options include augmented anatomic glenoid components, augmented reverse glenoid baseplates, and in-lay anatomic glenoid components. Partially due to these recent improvements, the use of shoulder arthroplasty has significantly increased. Between 1993 and 2007, there was a 319% increase in anatomic TSA procedures with annual increases between 6% to 13% (Day et al. 2010). More recent estimates project a ninefold increase in should arthroplasty procedures between 2011 and 2030 in the United States alone (Padegimas et al. 2015). Here, we will discuss recent innovations that assist in addressing these difficult deformities.
Glenoid Bone Loss in Primary Total Shoulder Arthroplasty
Glenohumeral osteoarthritis may lead to increased retroversion and posterior bone loss, specifically in the young male population. This presents an additional challenge which must be addressed at the time of surgery. Walch reported an average retroversion of 17.3° in patients with glenohumeral osteoarthritis (Walch et al. 1999) and later showed anatomic replacement in B2 glenoids results in higher rates of glenoid component loosening and more complications (Walch, Moraga, et al. 2012). The options for addressing this deformity include inserting the glenoid component in the patient’s native version, high-side reaming, stepped or wedged augmented glenoid components, bone grafting, or utilizing an in-lay glenoid component. The optimum treatment is currently not known.
Historically, increased retroversion and posterior bone loss was either ignored or the high side of the glenoid was reamed down to a flat surface. It is generally accepted that with <15° of retroversion, eccentric reaming can safely be performed to prevent placing the glenoid component in excessive retroversion while preserving glenoid bone stock (Wang et al. 2015). However, attempting to correct a deformity larger than this has been shown to result in bone loss from the anterior glenoid and medialization of the joint line with loss of subchondral support (Chen et al. 2017; Walch, Young, et al. 2012). For larger deformities, other options should be considered, including the use of an augmented glenoid component.
The posteriorly augmented glenoid component has been used to restore excessive retroversion without excessive glenoid reaming and medialization. Options include full wedge, half wedge, or stepped augmented components. A biomechanical study showed a significant decrease in bone removal when placing augmented components, with posterior half wedges requiring the least amount of bone removal for placement. (Knowles, Ferreira, and Athwal 2015). Multiple biomechanical studies have shown improved or equivalent stability of these components compared to standard implants. Iannotti compared four distinct glenoid component designs and found a stepped augmented component to have the least amount of anterior glenoid liftoff after 100,000 loaded cycles (Iannotti et al. 2013). Clinical studies have been favorable as well. A retrospective evaluation of 71 shoulders with B2 and B3 glenoids using an augmented glenoid component found improved clinical and radiographic outcomes at a minimum follow up of 2.4 years. However, patients with more preoperative retroversion and posterior bone loss were more likely to have incomplete correction of version and posterior humeral head subluxation (Ho et al. 2018). Priddy reported on 37 shoulders with a full wedge posterior augment in B2 and B3 glenoids compared to 37 control shoulders with standard implants and found no differences in radiographic lucencies or revision free survival (Priddy et al. 2019). Finally, a review of 21 patients with an average posterior bone loss of 4.7mm treated with an augmented component showed improvements in patient reported outcomes and range of motion. Additionally, no clinical or radiographic failures were encountered (Stephens, Spencer, and Wirth 2017). Long term clinical and radiographic outcome data is needed to confirm early results.
Case Example
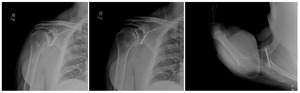
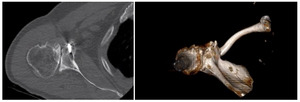
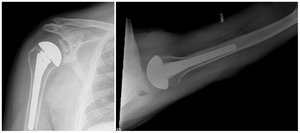
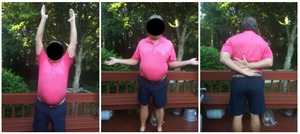
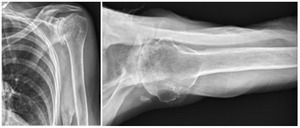
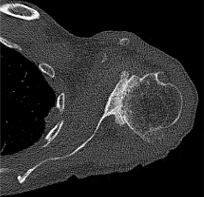
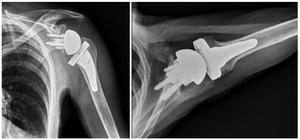
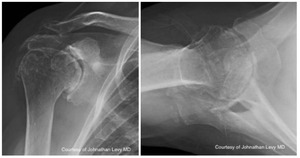
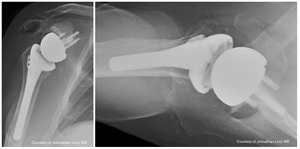
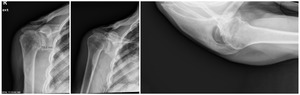
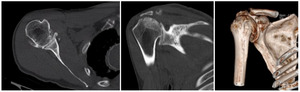
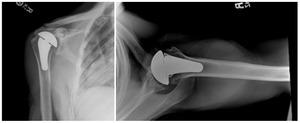
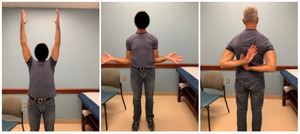
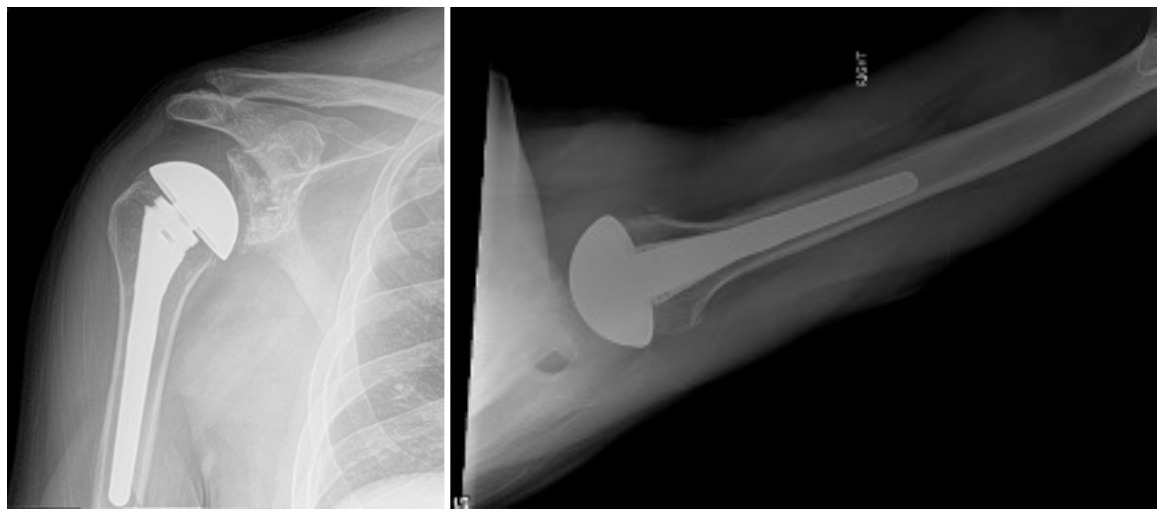
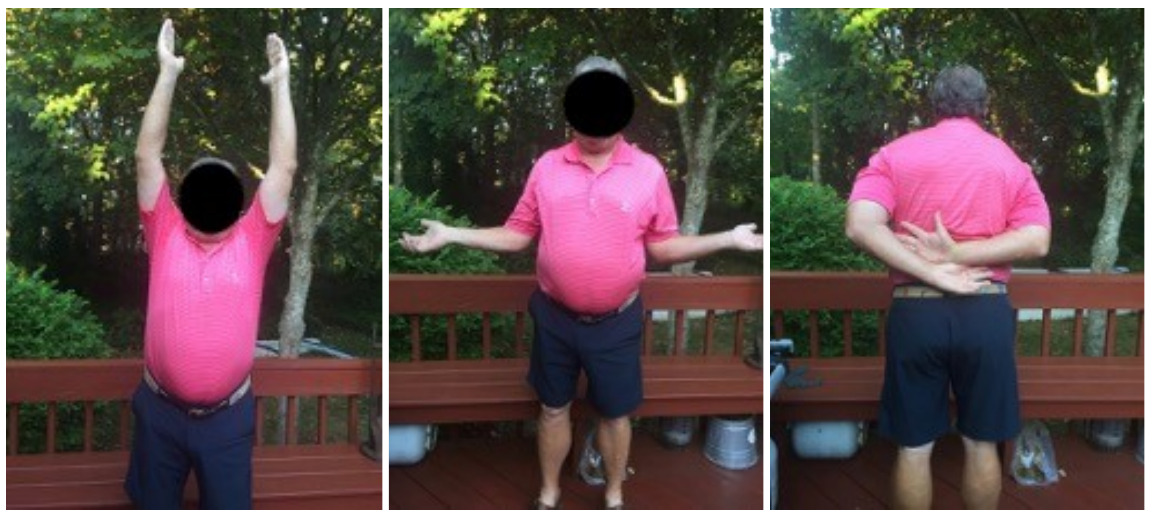
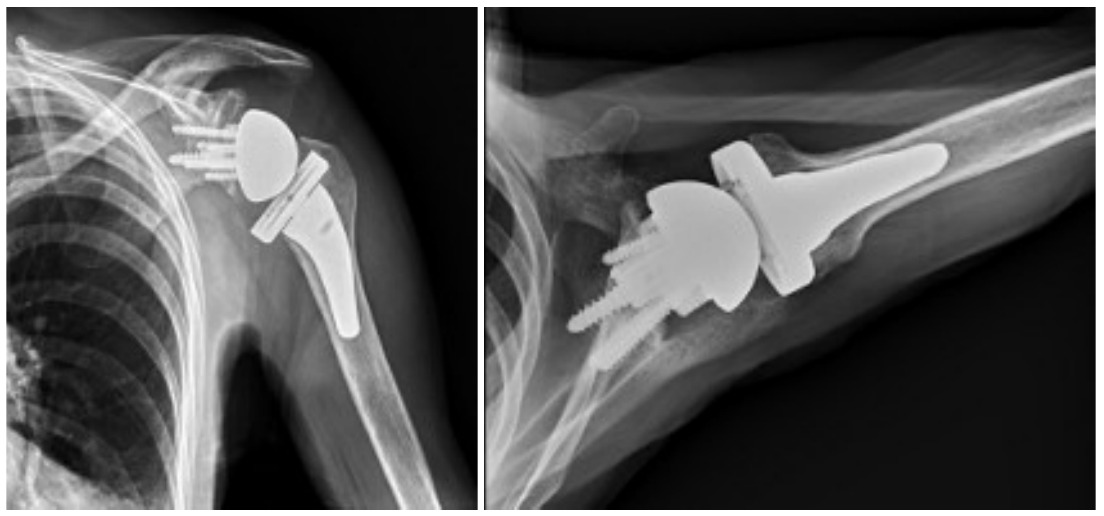
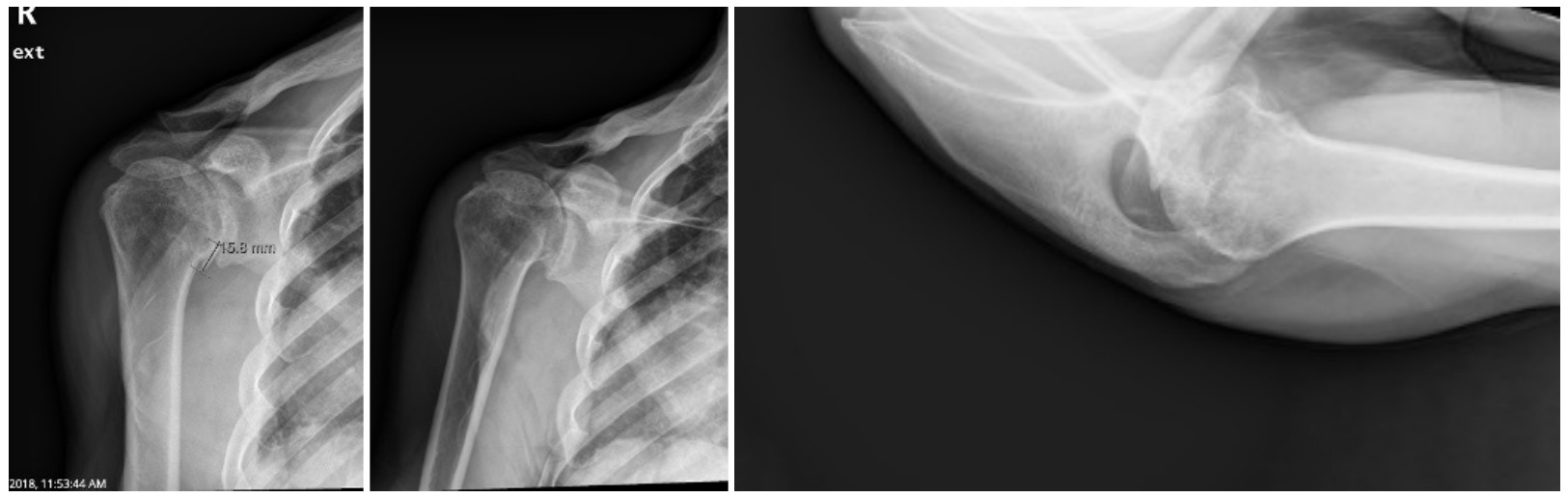
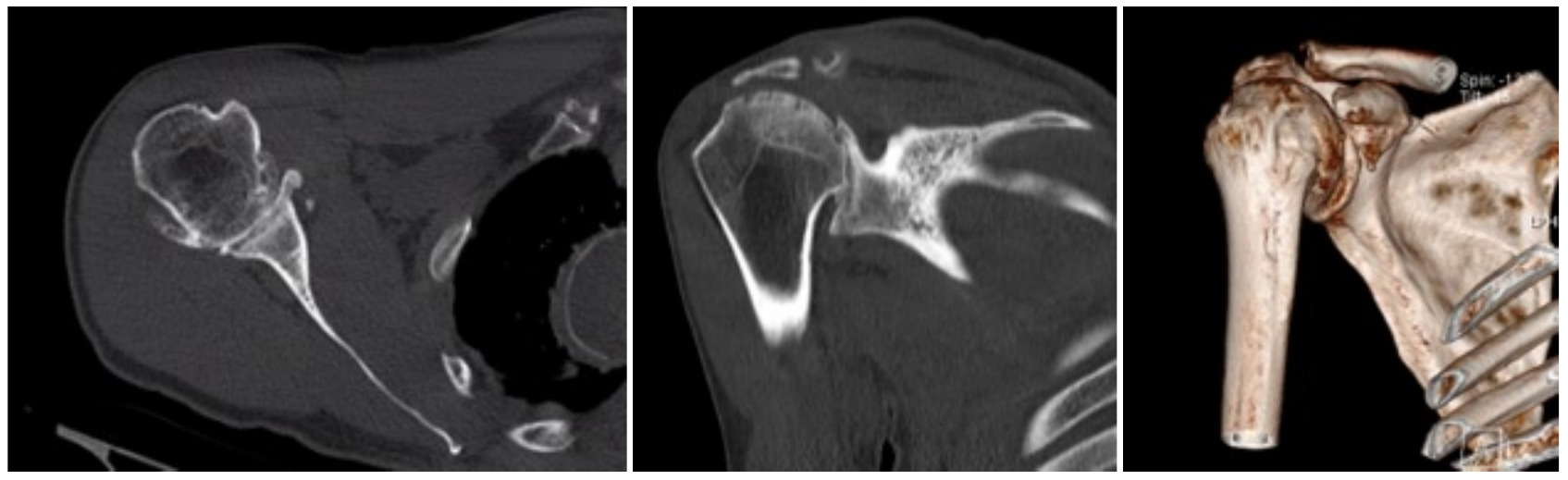
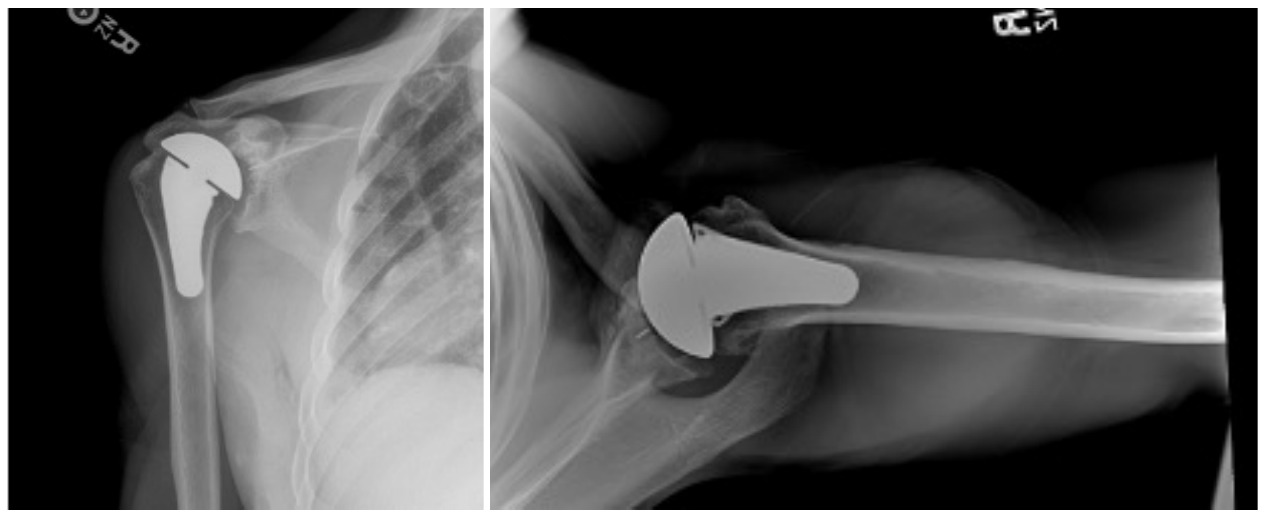
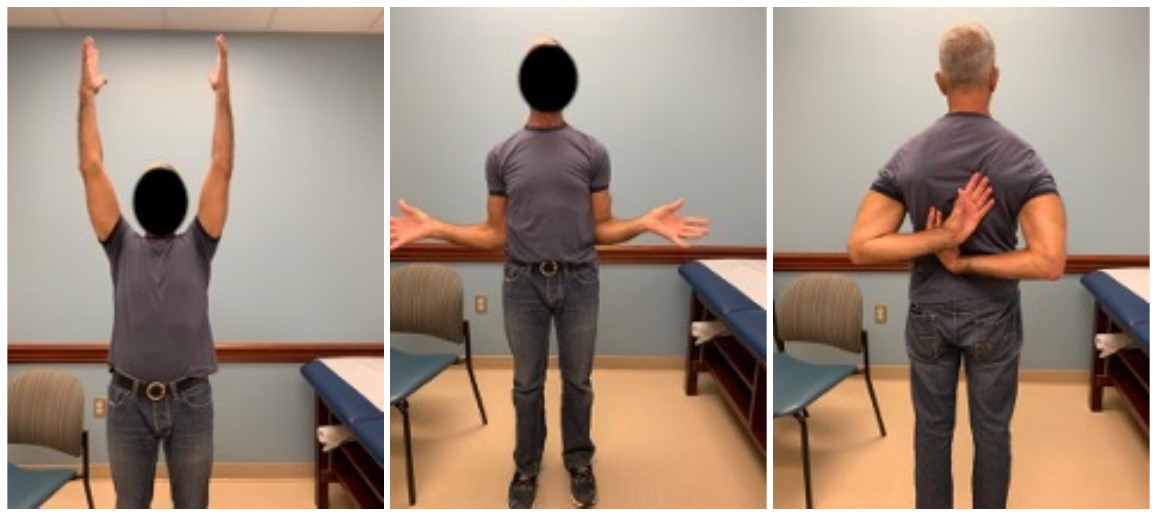
Case one is a 58-year-old male who is an avid golfer with long standing right shoulder pain. Surgical history includes previous Bristow procedure 30 years prior. Current Subjective Shoulder Value (SSV) is 30% and pain is 8/10 at baseline. Active range of motion was 110° of forward flexion, -10° of external rotation and interna rotation to the lateral buttock. Radiographs (Figure 1) and computed topography (CT) scan (Figure 2) show retained implants with loss of joint space, glenoid retroversion, and significant posterior humeral head subluxation. The patient had failed conservative management and was indicated for arthroplasty. Due to significant posterior bone loss and retroversion, an augmented anatomic component was selected for this patient. Radiographs at one year show a well seated glenoid component with restoration of the joint line and a centered humeral head (Figure 3). At five years, SSV had improved to 95% with excellent clinical motion (Figure 4).
Glenoid Bone Loss in Reverse Shoulder Arthroplasty
Similar to the anatomic TSA discussed previously, the RSA baseplate has seen multiple design innovations since it was first introduced in the United States in 2003. The original reverse ball and socket designs were mechanically flawed with inadequate glenoid fixation. This led to glenoid loosening and early catastrophic failure (Flatow and Harrison 2011). Second generation designs significantly improved the glenoid side fixation utilizing a central hydroxyapatite-coated post and peripheral screw fixation (Mourad et al. 2020). This led to significantly improved clinical outcomes (Cuff et al. 2008). As the RSA implant continues to be utilized for broader indications, new challenges have spurned new innovations.
Glenoid bone loss can also be encountered in reverse shoulder arthroplasty, in both the primary and revision setting, and needs to be appropriately addressed. Historically, allograft or autograft bone grafting has been the gold standard for addressing glenoid bone loss in RSA (Seidl, Williams, and Boileau 2016). While good outcomes can be achieved with this technique, unique complications can occur including graft resorption or graft fracture (Malahias et al. 2020). Additionally, donor site morbidity or lack of available autograft can be problematic. Due to these concerns, augmented baseplates have recently been developed.
A comparison study of 80 patients undergoing primary RSA with 39 receiving augmented components and 41 being treated with primary components and bone grafting found significant improvements in range of motion, pain, and function in both groups. However, there were six complications in the bone graft group and none in the augmented baseplate group (Jones, Wright, and Roche 2015). Virk evaluated the clinical and radiographic outcomes using an 8° posterior wedge baseplate in Walch B2, B3, and C glenoids. All patients experienced excellent clinical and radiographic outcomes with a low 4.5% complication rate at a mean follow up of 40 months (Virk et al. 2020).
While posterior bone loss is most commonly encountered, superior glenoid defects can be seen in patients with long standing rotator cuff deficiency and proximal humeral migration. Augmented components have also been utilized in this clinical setting. Liuzza retrospectively reviewed the use of augmented components for Favard type E1, E2, and E3 defects. Sixty-eight patients, with a mean follow up of 40 months, showed excellent clinical outcomes. Five complications were seen including one case of aseptic glenoid loosening (Liuzza et al. 2020).
Case Example
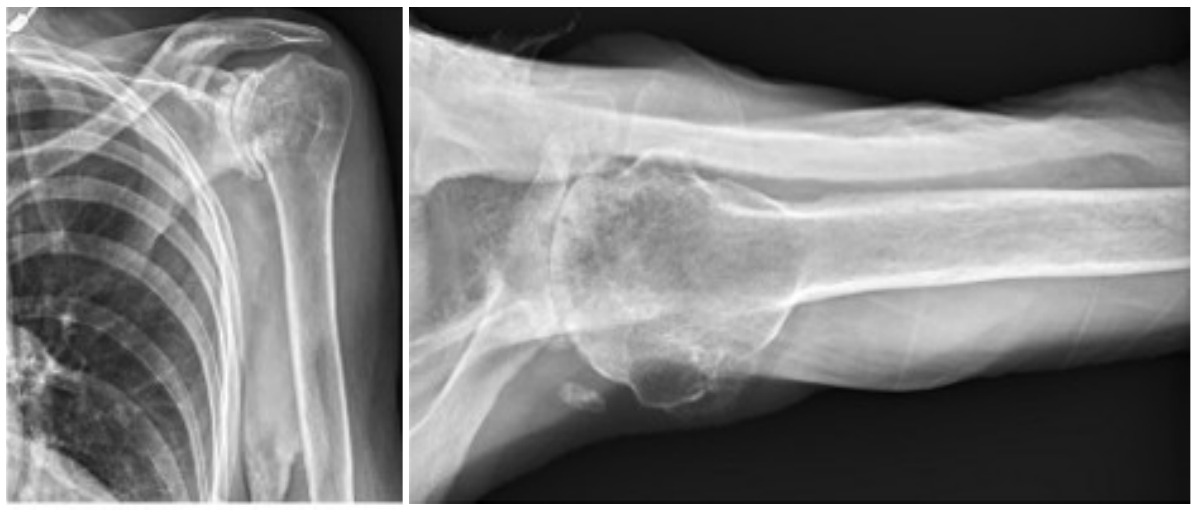
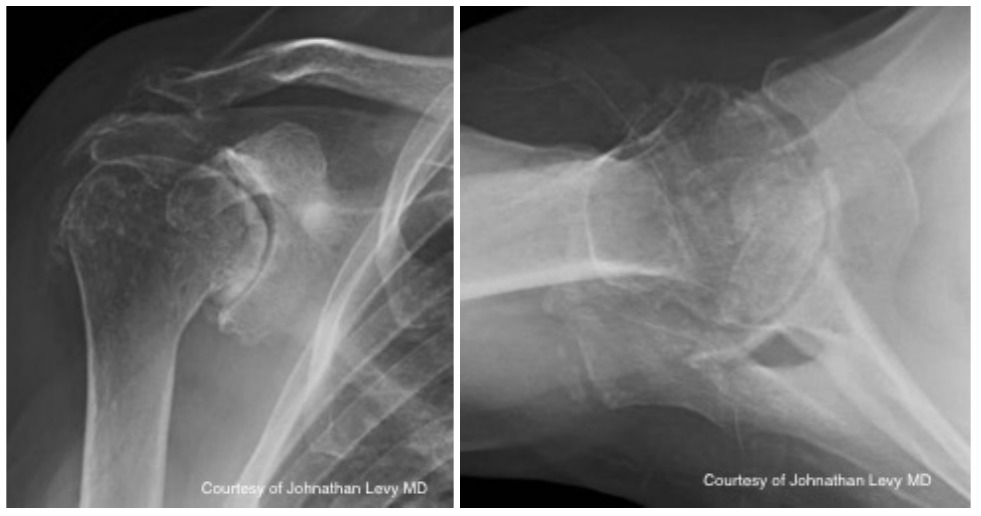
Case two is an 86-year-old female with long standing left shoulder pain and loss of function. Preoperative exam shows 70° of active forward flexion, neutral external rotation, and internal rotation to the lateral thigh. Radiographs (Figure 5) and CT scan (Figure 6) show significant posterior bone loss and retroversion with medialization of the joint line. Options include asymmetric reaming, an augmented component, or bone grafting. An augmented reverse baseplate was selected for this elderly female. Radiographs at six months show a well fixated baseplate with improvement of the pre-operative retroversion (Figure 7).
Glenoid Bone Loss in Revision Shoulder Arthroplasty
Significant bone loss can be encountered in the setting of revision arthroplasty. Options to address this have been limited. As discussed previously, bone graft, either autograft or allograft, was the previous gold standard though it does have its limitations. Augmented baseplates can also be used in the setting of mild bone loss. However, with large cavitary or uncontained defects other options must be considered.
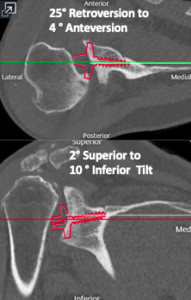
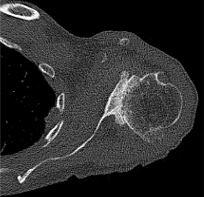
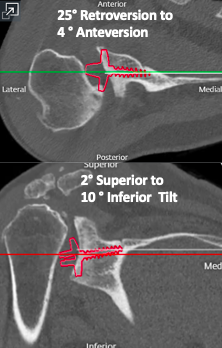
In traditional RSA, the glenoid baseplate should match the native glenoid version, with the center screw or post following the anatomic center line. However, in the setting of significant glenoid bone loss, utilizing this method may lead to insufficient backside contact of the glenoid baseplate and poor initial fixation. Frankle originally described the alternative center line technique which utilizes the bone in the central glenoid vault, where the scapular spine meets the coracoid base (Figure 8). This requires anteversion and inferior tilting of the glenoid baseplate and allows for enhanced baseplate fixation while sacrificing traditional anatomic placement (Frankle et al. 2009). This alternative placement of the baseplate does raise concerns regarding potential functional deficits and higher rates of scapular spine fractures (Colley, Polisetty, and Levy 2020). With an anteverted glenosphere, internal rotation may be limited. Also, utilizing the alternative center line may put increased stress on the scapula, leading to scapular spine fracture. However, in the setting of significant glenoid deformity, the alternative center line may be the only option for adequate baseplate fixation.
Clinical outcomes of the alternative center line have been reported. Klein reported a comparative study of 143 shoulders followed for two years. Fifty-six patients were treated with the alternative center line technique due to glenoid deformity. The authors found no difference in postoperative American Shoulder and Elbow Surgeons scores. There was one complication in the alternative center line group, which was a periprosthetic fracture after trauma (Klein et al. 2010). A similar study compared 22 patients using alternative center line technique with 66 age, sex, and indication matched control patients. There were no differences in clinical outcomes and no evidence of radiographic loosening in either group. Specifically, there was no difference in internal rotation between groups. However, there was a higher incidence of acromial stress fracture in the alternative center line group (9% vs. 3%) (Colley, Polisetty, and Levy 2020).
Case Example
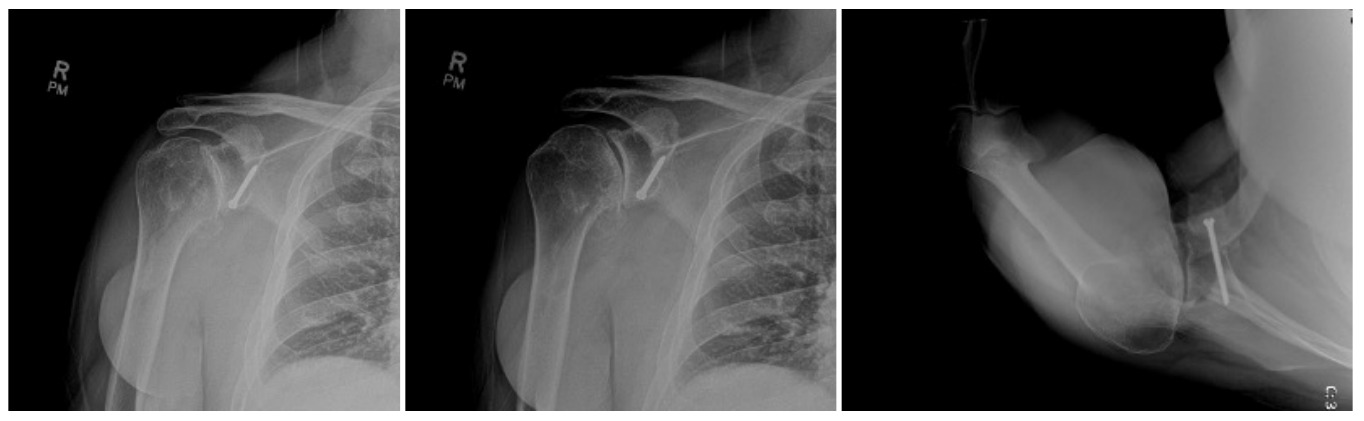
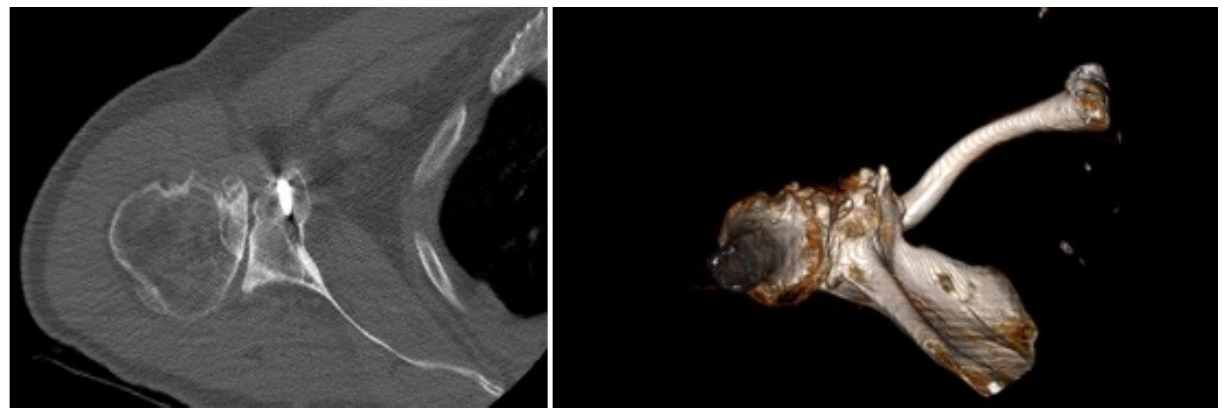
Case three is a 79-year-old male with cuff tear arthropathy with significant bone loss and medialization of his joint line (Figure 9). Three-dimensional imaging confirms these findings (Video 1). Due to the loss of posterior bone and available bone down the native glenoid vault, alternative center line was selected for this patient. Post-operative radiographs show a glenoid baseplate with exaggerated inferior tilt and anteversion, consistent with utilization of the alternative center line (Figure 10).
Glenoid Arthrosis in the Young, Active Patient
The younger population presents a unique challenge to the shoulder arthroplasty surgeon. This is due to the increased demands of the implant, concerns regarding longevity of the components, and the likely necessity of future revision procedures. Between 2011 and 2030, a 300% increase in arthroplasty is anticipated in patients under the age of 55 years old (Padegimas et al. 2015).
The most common long-term complication of anatomic arthroplasty is loosening of the glenoid component, which accounts for up to 24% of all complications (Gonzalez et al. 2011). Radiolucent lines are commonly seen around glenoid components, although these do not always lead to clinical symptoms or failure. According to a recent systematic review, radiolucent lines are common and occur at a rate of 7.3% per year (Papadonikolakis, Neradilek, and Matsen 2013). Due to these concerns, innovations in glenoid design have been developed in an attempt to decrease loosening in the young, active patient.
Traditional glenoid components are onlay designs and can either be pegged or keeled. Onlay components cover the entirity of the glenoid articular surface and require reaming of the glenoid face to match the backside contour of the implant. In contrast, inlay components are inset within a preserved rim of peripheral bone. Inlay components have recently been developed with the goal of limiting the amount of necessary glenoid reaming as well as improving long term survival. The inlay design allows for decreased micromotion due to the prevention of edge loading (Pinkas, Wiater, and Wiater 2015). The necessity of this innovation is due to the increased demand for arthroplasty in the younger population. Instrumenting the glenoid in a younger individual essentially guarantees the need for a revision procedure during that patient’s lifetime. Increasing the longevity of the primary glenoid component and limiting glenoid reaming can preserve precious glenoid bone stock. A biomechanical study showed decreased distraction of inlay components compared to onlay components with repeated dynamic loading. Displacement of the inlay component was 87% and 73% less compared to pegged and keeled components, respectively (Gunther et al. 2012). A subsequent cadavar study showed similar findings with no gross loosening of inlay components after 4,000 cycles of loading where onlay components loosened as early as 1,126 cycles (Gagliano et al. 2017). Clinical studies have also shown good outcomes and low rates of loosening or complications. Davis reported on nine shoulders with a minimum of two year follow up treated with an inlay glenoid component. Improvements were seen in range of motion, Single Assessment Numeric Evaluation (SANE) scores, and VAS pain scores (Davis et al. 2016). A more recent retrospective review of 27 shoulders with a minimum of two year follow up showed no radiographic loosening, improved clinical outcomes, 100% revision free survival, and a 92.6% rate of return to work. While biomechanical and early clinical results are promising, long-term outcomes data is needed.
Case Example
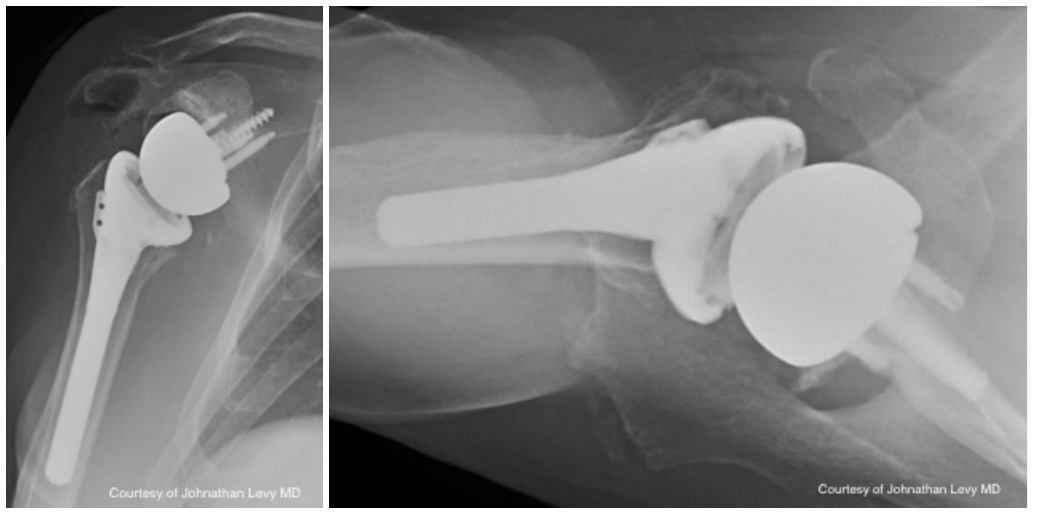
Case four is a 63-year-old retired nurse who was very active with weight lifting, horseback riding, tenis, and yoga. Surgical history included two prior arthroscopid debridements and capsular release. His pre-operative Subjective Shoulder Value (SSV) was 30% and pain score was 10/10. Exam showed 140° of active forward flexion, 30° of active external rotation, and active internal rotation to the sacrum. Radiographs (Figure 11) and CT (Figure 12) scan showed end stage arthritic changes without significant glenoid deformity. Options were discussed with the patient and due to his relatively young age and activity level, an inlay glenoid component was selected. This was combined with mild high side reaming. Radiographs at one year show a well seated component without signs of loosening or failure (Figure 13). Postoperative SSV was 95% and he returned to all prior activities (Figure 14).
Conclusion
There have been numerous advances in glenoid sided technology since Charles Neer implanted the first glenoid component almost 50 years ago. While failures do still occur, the glenoid side of shoulder arthroplasty is no longer the significant concern that it once was. As shoulder arthroplasty continues to become more commonplace, the burden of revision procedures will increase. With this, more advanced glenoid sided deformities will be encountered. Recent advances such as augmented components and the alternative center line technique will allow for improved clinical outcomes. Likewise, inlay glenoid components may provide a better option for the young, high-demand patient with end stage glenohumeral arthritis.