INTRODUCTION
Primary total knee arthroplasty (TKA) is a well-established elective surgical procedure in which optimum outcomes such as relief of pain and restoration of function are routinely achieved (Lange et al. 2017). The advancements in component technology, associated surgical instrumentation, adjunctive prophylactic peri-operative treatments, and operating room efficiencies have all greatly contributed to the routine success of the surgeon’s technical performance of this procedure (Small et al. 2013).
With the recent rapid shift of surgical cases involving increasingly complex interventions from main hospitals to ambulatory surgical centers (ASCs), the effect of same-day patient discharge to home within hours of surgery completion may affect the risk of surgical site complications (SSCs) and subsequent surgical site infections (SSIs) costs (Crawford et al. 2020). In addition, reduction of the ASC medical staff’s time for early surgical site incision management is a major area of post-operative concern (Wilson et al. 2023; Law et al. 2020). Regardless of the location of surgery (main hospital or ASC), or the surgery performed, it is reported that surgical site complications (SSCs) and subsequent surgical site infections (SSIs) costs the United States healthcare system an estimated $3B to $10B annually ((CDC) UCfDCaP 2024; Ban et al. 2017), with approximately 60% of all SSIs being preventable (Ban et al. 2017).
In an effort to address potential post-operative SSCs, the mechanisms of action and benefits of negative pressure wound therapy (NPWT) are becoming better known for closed incision wound care across many disciplines of surgery (Lancerotto, Bayer, and Orgill 2012; Tanaydin et al. 2018; Ma et al. 2016, 2017). NPWT is becoming increasingly used as an adjunctive prophylactic wound-care mechanism for closed surgical incisions, with positive efficacy independent of surgery type or location of the surgical incision. Recent publications demonstrate that the delivery of NPWT for any surgical incision, significantly contributed to minimizing wound tension and stimulation of vascular perfusion at the surgical incision (Lancerotto, Bayer, and Orgill 2012; Ma et al. 2016, 2017). This leads to increased surgical site oxygenation which enhances the rate of healing and significantly reduces the incidence of SSCs and SSIs (Lancerotto, Bayer, and Orgill 2012; Ma et al. 2016, 2017). The reduction of SSCs and SSIs, using Negative Pressure Wound Dressings (NPWD) for the delivery of NPWT, yields an economic effect which significantly decreases the healthcare cost burden across all surgical procedures (Nherera et al. 2021).
However, despite the well-known benefits of the delivery of NPWT, in conjunction with surgeon understanding and acceptance, the use of externally powered NPWDs is slow to be incorporated into various surgical standards of post-operative care. Multiple reasons exist for the low acceptance of externally powered NPWD use, including medical staff frustration with patient education and device serviceability, further patient frustration and reduced compliance leading to interruptions in use affecting the delivery of NPWT, and payor increased cost perceptions. To address the issues of acceptance, compliance and continuous delivery of NPWT, any developments in NPWD should incorporate into the dressing a non-tethered, non-powered mechanism for applying uninterrupted NPWT while minimizing any issues of acceptance, patient compliance and cost. Therefore, we introduce the use of a self-contained, non-powered, NPWD for the continuous delivery of NPWT, and its initial application in primary TKA. The purpose of this case series review was to report on the feasibility, patient compliance, and results of the use of this NPWD system for primary TKA.
MATERIALS AND METHODS
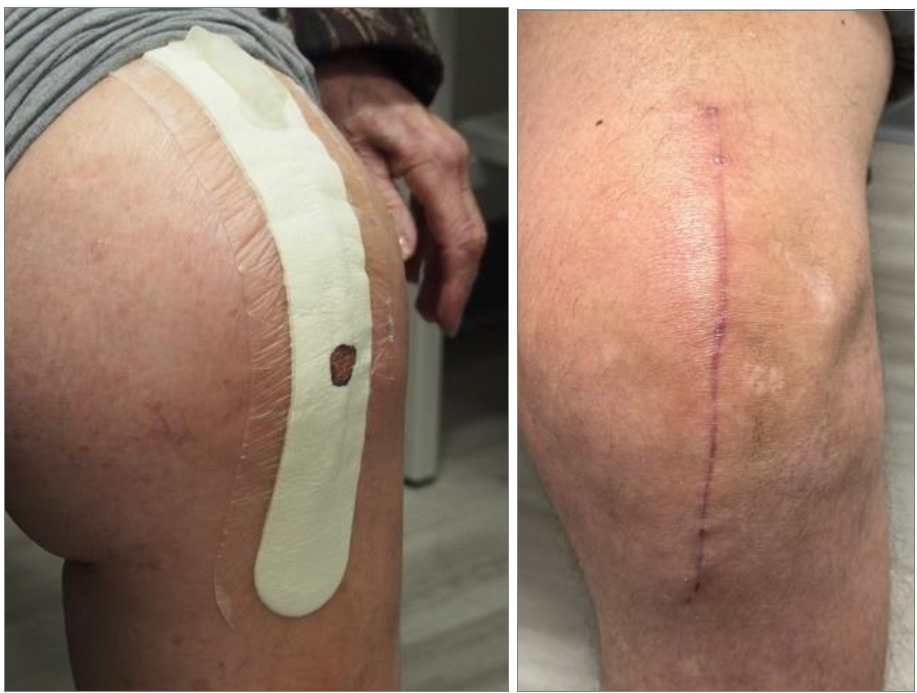
In lieu of the various issues as seen with currently used NPWDs (i.e.: Prevena™ Solventum/3M/KCI, PICO™ Smith & Nephew), beginning in August of 2024, the senior author implemented the use of a non-externally powered, NPWD on all patient candidates on primary TKAs for the delivery of continuous NPWT (NPseal®, Guard Medical, Miami, FL) (Figure 1). The NPseal NPWD is an FDA 510K cleared device intended for single use in the delivery of a continuous negative pressure therapeutic range between -75mmHg and -125mmHg through 7 days post-operatively. The NPseal NPWD is a self-contained NPWD composed of a dressing pad of varying lengths to match the surgical incision length, a proprietary frame-liner adhesive technology for ensuring a robust airtight seal across articulating joints, and a simple pinch pump that allows the patient to independently monitor and maintain negative pressure within the therapeutic range without the use of a powered pump. The pinch-pump incorporates a pump/valve mechanism which includes a one-way valve for the removal of air from the dressing-to-skin interface, and an outlet valve to remove the air from the pump mechanism. The pump is engaged using a finger-to-thumb pinching and the therapeutic range of negative pressure is achieved when the pinch-pump collapses and holds an “hourglass” shape. Patient monitoring requires observing if the pump remains collapsed. If the pump begins to decompress, the patient is instructed to re-apply the pinching (usually 3-5 pinches) to maintain the previous “hourglass” shape. We instructed patients to contact the office if more than 50% of the dressing became saturated with exudate from the wound.
A retrospective review of patients that received the NPseal NPWD following TKA was initiated and a consecutive, non-selected, case series of the first 100 patients beginning August 28, 2024, were summarized. All patient information was de-identified and only information pertaining to basic demographics (gender, age, side, pre-operative co-morbidities), NPWD feasibility, patient compliance, and results regarding any adverse or serious adverse events was collected. Pre-operative patient comorbidities were tallied to identify those medical issues that adversely affect wound healing, and the effectiveness of NPseal for avoiding related SSCs, and especially subsequent SSIs.
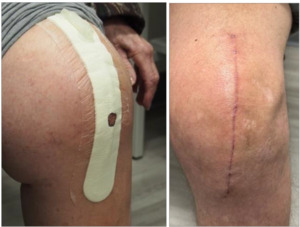
All surgeries were performed by senior author (AS) through a minimally necessary midline skin incision, and a medial parapatellar capsular incision. Following wound closure, all patients received the NPseal NPWD surgical dressing and were trained on the use and maintenance prior to same-day discharge to home. The dressing was removed at the first post-operative follow-up, and the wounds were inspected (Figure 2).
Descriptive statistics (average, standard deviation, data range) were used to summarize the group characteristics. When applicable groups were identified, the Student’s T-Test was applied, and with three or more groups, a one-way analysis of variance (ANOVA) was applied. In all cases, a two-tailed significance P-value < 0.05 was used.
RESULTS
Of the first 100 consecutive, non-selected, primary TKA patients, all patient charts were available for review and follow-up visits completed. Between August 28, 2024, and February 19, 2025, there were 66 females (66%) and 34 males (34%) with a combined average age at the time of surgery of 72.9 ±8.3 years (range: 52.8 years to 89.5 years). There was no statistical difference between average female versus male age (p = 0.105), with an average age of females only was 73.8 ±7.9 years (range: 57.1 years to 87.6 years), and for males only 71.0 ±8.8 years (range: 52.8 years to 89.5 years). The combined average patient body mass index (BMI, kg/m2) was 29.8 ±4.6 (range: 19.0 to 41.0), and for females only was 29.1 ±4.8 (range: 19.0 to 41.0), and for males only was 30.2 ±4.3 (range: 22.8 to 38.0), and when compared, were not statistically different (p = 0.231). Patient demographics are summarized in Table 1.
There was a total of 267 comorbidities identified for all 100 patients (3.2 ±1.3, range: 0 to 6). Of the patients that presented comorbidities, the following were singled out; hypertension (HT) n=67, hyperlipidemia (HDL) n=37, cancer (CA) n=27, other cardiac (OC) n=23, and DM-II n=20. All comorbidities reported are summarized in Table 2. With the absence of post-operative SSCs, there was no effect of adverse wound events / SSCs related to specific comorbidities or comorbidities in combination.
Within the review of the surgical notes, we found that there were three (3) distinct surgical incision closure techniques used prior to application of the NPWD. Between August 28, 2024, and October 09, 2024 (n=26), the surgical incision was close with interrupted subcutaneous sutures with Dermabond™ Prineo™ topical liquid skin adhesive with a self-adhering mesh (Ethicon, Inc., Raritan, NJ). Between October 10, 2024, and November 19, 2024 (n=23), the surgical incisions were close with a continuous subcutaneous suture and Dermabond™ topical liquid skin adhesive (Ethicon, Inc., Raritan, NJ). And, between November 20, 2024, and February 19, 2025 (n=51), closure returned to the use of an interrupted subcutaneous suture with staples.
All patient queries during the initial post-operative follow-up were similar regarding the “ease of use” and patient compliance with monitoring and maintaining the negative pressure gradient during recovery. This was evident with the pump being engaged with the “hourglass” shape intact at follow-up. Upon visual assessment, all wounds were found to be progressing without evidence of any SSCs. However, there were three patients identified with post-operative adverse events. One patient noted early post-operative nausea and vomiting (PONV) which resolved without intervention. Two other patients (1, 82 y/o female; 1, 64 y/o male) upon follow-up and dressing removal, had extensive skin reactions which has been linked of contact dermatitis (CD), and resolved without further issue using an over the counter, 1% topical hydrocortisone cream and oral Benadryl® (Johnson & Johnson, New Brunswick, NJ). Both patients were in the closure group utilizing Dermabond in conjunction with a continuous subcutaneous suture. From these two cases, the lead author (AS) immediately discontinued the use of Dermabond and switched to the use of interrupted subcutaneous sutures and staples. Overall, we found for these first 100 patients reviewed, age, gender or pre-operative comorbidities had no adverse correlation with the current state of assessed wound healing.
DISCUSSION
We report the results of a single surgeon’s first 100 consecutive, non-selected TKA case series involving a single use, non-externally powered, NPWD which continuously delivers NPWT. The use of the NPseal NPWD is well established across other surgical disciplines and has recently been introduced for use across orthopedic surgical applications (Cagney et al. 2020; Fukuzaki et al. 2022; Grauhan et al. 2013; Roos et al. 2021; Wang et al. 2025). We found that the use of the NPseal NPWD is feasible in primary TKA across all patient types, independent of pre-operative single or multiple patient co-morbidities that generally contribute to delayed and poor surgical incision healing and subsequent SSCs.
Much evidence exists for the medical and cost benefits of NPWT as an adjunctive surgical incision treatment modality across multiple surgical disciplines including (but not limited to) abdominal (Roos et al. 2021; Wang et al. 2025), cardiovascular (Grauhan et al. 2013), and plastics (Cagney et al. 2020) procedures. In a meta-analysis (MA) of 57 randomized controlled trials (13,744 patients), Groenen, et al, designed the MA to include the primary endpoint of the difference of NPWT versus standard dressings: gauze-based dressings with or without silver, hydrocolloid-based dressings or silicone gauze, in the prevention of SSI (Groenen et al. 2023). From the MA review, the authors reported with high certainty the effectiveness of “NPWT over standard dressings in the prevention of SSIs.” Secondarily, the benefit of wound dehiscence and seroma avoidance were shown to be highly significant with NPWT versus standard dressings. In another meta-analysis modeling the benefit of NPWT in the reduction of SSIs and the projected cost effectiveness/savings of NPWT in the US and UK healthcare systems (Nherera et al. 2021). Nherera, et al, reported that the use of NPWT significantly decreases the incidence of SSC and subsequent SSI across all closed surgical incisions included for study, thus leading to significant healthcare cost savings by reducing post-operative SSIs at the surgical incision site (Nherera et al. 2021). Through this observational review of 100 primary TKA patients, we found that the benefits of NPWT in the avoidance of SSCs were possibly achieved through the use of the self-contained, non-powered, continuous NPWD reviewed.
Currently available NPWDs commonly utilize a powered unit that either tethers the patient to a wall power outlet or requires wearing a battery powered unit thus decreasing surgeon use and frustrating patients with post-operative compliance. We introduce the use of a self-contained, non-powered, continuous NPWD for primary TKA, which allows the patient to maintain NPWT compliance and their post-operative rehabilitation schedule and level of independence while easily monitoring and maintaining the known therapeutic range of NPWT.
For all surgical procedures, patients presenting with multiple, significant comorbidity factors are considered “high-risk” for an increased incidence of SSCs and subsequent SSIs. Nherera, et al, found that when assessing those patients that present with “high-risk” comorbidities, this MA subgroup benefitted significantly greater from the use of NPWT, with greater improvement in outcomes at reduced costs and identifying NPWT as the dominant contributing treatment across a larger proportion of patient (Nherera et al. 2021). Our patient group presented with an array of comorbidities however, we did not observe any AEs associated with wound healing or significant objective assessment differences of wound healing.
There were three AEs reported during this review. One “minor” AE was possibly due to a reaction to anesthesia and resolved with issue. However, two patients presented with evidence of contact dermatitis following wound sealing with Dermabond topical liquid skin adhesive prior to the application of the NPWD. A significant post-operative skin reaction in both patients, extending beyond the NPWD was noted at the most recent follow-up. Following the use of Dermabond, similar adverse skin reactions have been reported in the Manufacturer and User Facility Device Experience (MAUDE) database. In 2021, Lee, et al, reported a summary of 29 adverse events following the use of Dermabond in which the two main AEs reported in 20 (69%) patients identified as CD, and wound dehiscence in 4 (14%) (Lee et al. 2022). Other case reports have reported similar findings within the early post-operative period in patients with no known allergies (Howard and Downey 2010; Nigro et al. 2020). The occurrence of a similar rash in the two patients reported from our observations prompted the lead author (AS) to discontinue the use of Dermabond as a topical application to surgical wounds. Following the use and discontinuation of both Dermabond™ Prineo™ (closed with interrupted subcutaneous sutures and NPWT) (n=26), and those with Dermabond™ (closed with continuous subcutaneous sutures and NPWT) (n=23), these patients were compared to the remaining patients with interrupted subcutaneous sutures and NPWT only (n=51). We found no adverse results related to surgical wound healing in the recent group without Dermabond™products used. While these are small comparative numbers, our closure technique has not changed since discontinuing the use of either Dermabond™ Prineo™ or Dermabond™ alone, and continues without reported adverse events related to surgical wound healing, and find that these closure products are not necessary for use with NPseal.
We acknowledge there are a few limitations of this patient review. This is a retrospective, single-surgeon review of patients which may inherently include a bias towards procedural performance and peri-operative care, which may exclude variations possibly observed with multi-centered clinical trials. We initiated this observational effort knowing the limitation of patient selection bias and decided to retrospectively review a non-selected sequence of the first consecutive 100 primary TKA patient set to receive the NPWD. Additionally, in an effort to maximize the effect of the treatment studied, and avoid inevitable patient related adverse events, case series will unavoidably exclude the “high-risk” patient population. Due to the non-selected sequence of the first 100 primary TKA patients, we included patients independent of comorbidity type and the number of combined co-morbidities that may have significant adverse effects on the success of wound healing and subsequent unanticipated early follow-up care.
CONCLUSION
The use of NPWT is well established across multiple surgical disciplines yet has been cautiously used across various orthopedic surgical applications. While the benefits of NPWT are also realized in orthopedics, devices are routinely driven by externally powered pumps which decease patient compliance and possibly interrupt the delivery of NPWT. In addition, the costs associated with these systems and their maintenance tend to push the use of these devices away from much of the surgical population and towards “high-risk” patients. From our observational review, and independent of patient pre-operative comorbidities, we are confident that the use of the self-contained, non-powered NPseal NPWD is feasible in primary TKA. We also conclude that the purpose of this retrospective observational study was successfully met (device application, patient compliance, general wound outcome, and patient satisfaction). Further use and study of the NPseal NPWD in primary and revision THA and TKA, and across other orthopedic applications, is warranted and should also focus on the monetary benefits to the total healthcare system in reduction of SSCs that burden the costs associated with ongoing treatment of related adverse events.

_25cm__single_use__self-contained__non-extern.png)
_25cm__single_use__self-contained__non-extern.png)