INTRODUCTION
Cartilage lesions of the patellofemoral joint are common and difficult to treat, due to the poor intrinsic healing potential within the tissue, the complex biomechanical environment of the extensor mechanism, and the significant forces experienced within this compartment during weight bearing (Gomoll, Minas, Farr, et al. 2006; Maffulli et al. 2011). Our understanding of articular cartilage structure, biochemistry, and biomechanics has significantly improved, but patellofemoral chondral lesions are still a challenge for both the patient and the treating surgeon. They are present in a wide segment of the population, they may be silent for years, and at some point, they will cause pain and impairment that may result in surgical interventions (Andrade et al. 2019; Ciccotti et al. 2012; Kobayashi et al. 2016).
Knee surgeons and sports medicine specialists have limited tools to precisely determine the degree of early patellofemoral cartilage damage (Ciccotti et al. 2012). Anatomical diagnostic images such as radiographs, CT scans or MRIs provide detailed information of articular contours and patellar position useful to determine joint stability, and helpful to define our therapeutic pathways (Dejour, Walch, Nove-Josserand, et al. 1994; Fulkerson 1983; Kohn, Sassoon, and Fernando 2016; LaPrade et al. 2018; Wang et al. 2012; Mattila et al. 2012). However, conventional diagnostic images offer limited information on patellofemoral cartilage and are commonly used only in symptomatic patients (Figueroa et al. 2007; Gomoll et al. 2011; Hart et al. 2017).
Patellofemoral chondral damage may occur with no pain or symptoms, and many subjects are never tested with any diagnostic protocol. Independent from subluxation or instability, therapeutic decisions on patellofemoral cartilage damage are often based upon subjective values such as pain or discomfort, supported with static indirect parameters like the size of the patellofemoral space in any given image (Kobayashi et al. 2016; Maffulli et al. 2011; Gomoll et al. 2011). This represents a weak medical diagnostic practice and leaves no room for preventive cartilage damage detection.
Sound emissions from the knee have long been investigated as a noninvasive diagnostic modality for assessing joint pathology, including cartilage damage (Abbott and Cole 2013; Madeleine et al. 2020; McCoy et al. 1987). Early efforts began in the late 19th and early 20th centuries, with pioneers such as Blodgett and Oehl using stethoscopes and early microphones to capture joint crepitus (Blodgett 1902; Oehl, Bohnenberger, Heinkelmann, et al. 1974). With the advancement of signal processing, piezoelectric sensors, and data analytics, modern vibroarthrography has demonstrated the ability to detect joint friction patterns (Cai et al. 2012; Andersen, Arendt-Nielsen, and Madeleine 2018; Kręcisz and Bączkowicz 2018). Despite these technological advances, clinical adoption has been limited due to variability in signal acquisition across different joint compartments and poor anatomical specificity (Franke, Dörner, and Schalwbe 2004). We believe that by focusing exclusively on the patellofemoral joint—rather than the entire knee—audioarthrography can achieve greater diagnostic precision and clinical relevance.
Joint sound emission research has helped understanding the sound patterns that can be acquired from the knee joint, and how to obtain them (Jeong et al. 2018; Madeleine et al. 2020; Teague et al. 2016; Töreyin et al. 2016). However, the clinical indications and applications have been quite unspecific, using data to document pathologies from tibiofemoral, meniscal, ligament and even bone sound patterns (Franke, Dörner, and Schalwbe 2004). In many ways, very sophisticated sound engineering systems have been used to determine in the knee is sick or healthy, but no specific decision-making patterns have been described (Madeleine et al. 2020). The specificity and sensitivity of modern diagnostic images are better to determine anatomical and biomechanical lesions or abnormalities of the knee (Bodelle et al. 2016; Van Eck et al. 2017). Sound emission analysis could be a functional dynamic test to provide valuable information on joint friction and function, that could be helpful in femorotibial conditions, but would probably be decisive in patellofemoral cartilage damage assessment.
To our knowledge, there are no reports regarding specific patellofemoral joint acoustic emission analysis. Furthermore, the correlation between sound emissions and the anatomical cartilage damage in vivo has not yet been reported. In the present study we aim to compare the arthroscopic patellofemoral cartilage findings to preoperative patellofemoral audioarthrography tests (PFAA) and to preoperative magnetic resonance images (MRI), in order to establish their accuracy, sensitivity, specificity and predictive values.
METHODS
One hundred and twenty-four volunteer patients that were indicated for arthroscopic meniscal or anterior cruciate ligament surgeries were included in this study. We excluded patients requiring an arthroscopy for patellofemoral conditions or any other knee problem. An international multicenter diagnostic clinical performance trial was done, including patients from five recognized international knee surgery clinics in USA, Portugal, Spain, Colombia, Ecuador and Chile. All patients signed an informed consent and were tested for patellofemoral sound emissions in the preoperatory period with a PFAA device. Our study included only patients with a preoperative standard MRI ordered for their meniscal or ligament lesions. We excluded patients with special diagnostic images for patellofemoral cartilage and did not require supplementary tests for the present study.
Arthroscopic ICRS (a-ICRS)
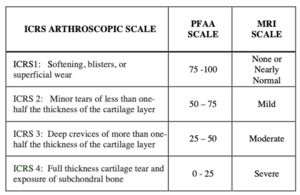
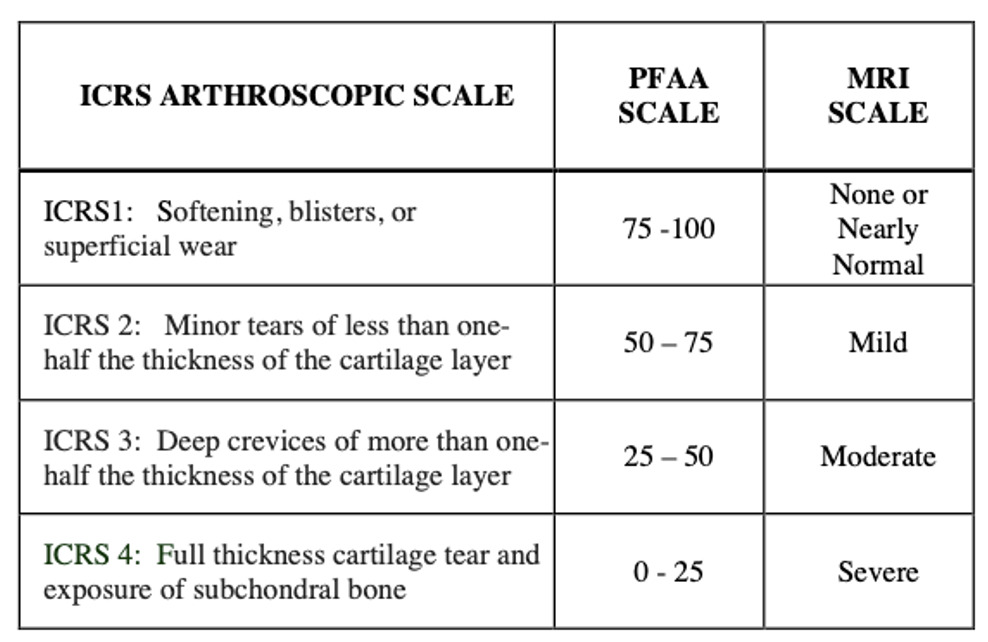
The arthroscopic procedures were performed without any changes in the standard treatment protocols and no extra time was added to the procedure. Arthroscopic surgery protocols involved cartilage evaluation including the patellofemoral joint. For the present study, the authors documented the arthroscopic view of the cartilage surfaces of the lateral and medial patellar facets, as well as the trochlear groove, and recorded the findings in a pre-established written form, using the validated International Cartilage Repair Society (ICRS) cartilage damage score (a-ICRS) (Figure 1).
Magnetic Resonance Imaging ICRS (MRI-ICRS)
We collected the preoperative MRI tests of all patients, as this is a standard study in meniscal and ACL surgical procedures. Conventional axial T1-T2 MRI sequences were reviewed, and no other special image software or contrast media studies were required. We analyzed the radiologist’s reports regarding patellofemoral cartilage, usually informed as mild, moderate, or severe damage, and converted this information in equivalence to the ICRS score (MRI-ICRS) (Figure 1).
Patellofemoral Audioarthrography ICRS (PFAA-ICRS)
An experimental PFAA device (KV032, KneeVoice inc. Los Angeles CA) was used to acquire and record the sound emissions of the patellofemoral joint in the preoperative period (Video 1).
This device had a piezo-contact microphone able to sense audio vibrations through contact with the skin. The contact microphone was positioned in the center of a specially designed semi rigid casing that isolates external sounds and prevents excessive pressure on the knee. The contact microphone was attached to a neoprene soft brace secured with Velcro tapes to the subject’s knee and was equipped with two anterior accelerometers placed over the distal thigh and proximal leg. These accelerometers were not in direct contact with the skin and were used to obtain positioning information of flexion and extension during the test. The contact microphone had an output of 106 +/- 3 dB, an impedance of 2.0 kOhms, a capacitance of 80 nF, and a max input voltage of 10 Vrms. The accelerometers contained a 3-axis gyroscope inside able to measure several motion related parameters such as angular displacement, acceleration, velocity, and orientation. In the PFAA device used for the present study, we measured flexion and extension simultaneously interrelated with the acquired sound emissions.
The contact microphone was positioned with the soft brace over the center of the patella in 30 degrees of knee flexion, and was connected to a computer with a specific data acquisition program that received the sound emission signals and the flexion-extension position of the knee (Figure 2). Demographic and clinical data, including gender, height, weight, laterality, pain scale, symptoms, physical activity, previous surgeries or interventions was uploaded using a touch screen (Figure 3-A). Patients were asked to perform eight non weight bearing 90 degrees
flexion-extension active movements, sitting on the edge of a stretcher. The PFAA system screen guides the patient with instructions of pace and number of repetitions, with the help of a live motion image of the knee (Figure 3-B,C). The computer is connected via internet to a neural network cloud database that categorizes all this information in order to provide a precise digital sound emission score. The score and graphics were displayed on the screen, and the acquired sound was reproduced on a speaker (Figure 3-D). The PFAA scores ranged from 0 to 100, being 0 the loudest and 100 the quietest knee. In order to match the four levels of ICRS arthroscopic chondral damage grading, scores were divided into four score groups (PFAA-ICRS).
The present report is a prospective diagnostic clinical performance trial and was statistically defined as a paired-design study that compared indirect information of an anatomical measurement: patellofemoral cartilage damage. Subjects were selected consecutively in the order of presentation among those meeting inclusion criteria. Categorical variables were summarized in terms of absolute frequencies and percentage. Continuous variables were analyzed in terms of mean, median, SD and interquartile range. The concordance of arthroscopic findings, ICRS’s expected score with PFAA or MRI were assessed using a weighted kappa index. The diagnostic accuracy of the PFAA was evaluated through the calculation of the area using arthroscopic ICRS findings as a reference. The area under the receiver operating curve (ROC) curve, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and probability ratio were calculated. For statistical purposes, patellofemoral cartilage damage was defined as an arthroscopic ICRS score of 2 or higher. The breakpoints for PFAA values were selected as those with the highest sensitivity/specificity value. These statistical analyses were performed with the Stata version SE 13.0 program.
RESULTS
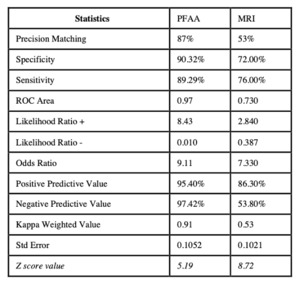
Thirty four patients did not complete the inclusion criteria and were excluded from the study. We analyzed 90 knees from 90 patients, 57 male (63%) and 33 female (37%), with an average age of 50.37 years (SD 15.38) and an average Body Mass Index (BMI) of 28.84 kg/m2 (SD 4.79). Age, gender and body mass index were Gaussian distributed. Sixty-three patients (70%) had some degree of cartilage damage diagnosed by arthroscopy. The arthroscopic ICRS scores were compared with the PFAA-ICRS and MRI-ICRS scores to determine accuracy. The scores matched in 78 patients when compared with PFAA (87%), and in 48 patients with MRI (53%). Accuracy was better with PFAA than MRI in 33% of patients with arthroscopic ICRS 1, 38% in ICRS 2, 34% in ICRS 3 and 15% in ICRS 4 (Figure 4).
Younger patients had better arthroscopic ICRS scores, as well as PFAA-ICRS and MRI-ICRS scores. Patients under 25 years of age had an a-ICRS average of 1.13 that increased almost linearly to end in an average ICRS score of 2.8 in patients over 65 years of age (Figure 5). Both PFAA-ICRS and MRI-ICRS scores increased accordingly with age, and the differences with arthroscopic findings were smaller with PFAA in all groups. No statistically significant differences were observed between a-ICRS and PFAA-ICRS in any age group (p=0.84) There was a significant difference of 0.62 between a-ICRS and MRI-ICRS only in the 35-45 years of age group (p=0.032).
The differences between PFAA-ICRS and MRI-ICRS were statistically significant in the 35-45 years of age group (0.54: p=0.028) and in the over 65 years of age group (0.46: p=0.042). Continuous scores were obtained for PFAA-ICRS, and the ROC analysis showed an area under the curve of 0.97. After determining the diagnostic accuracy of PFAA, a cut point analysis was performed in order to find the value with the highest sensitivity/specificity ratio. The cut point of greatest sensitivity/specificity was found with a PFAA value lower than 55, with sensitivity values of 90.32% and specificity values of 89.29%, along with a percentage of correct performance prediction of 95.40%. MRI had a sensitivity of 70% and a specificity of 76%, being 18.32% and 13.39% lower than PFAA respectively (Figure 6).
DISCUSSION
We found a significant equivalence between the patellofemoral cartilage damage diagnosed by arthroscopy and by PFAA. The diagnostic accuracy level of 87% is extremely favorable, particularly when compared to 53% obtained with MRI, the current non-surgical diagnostic gold standard. Analyzing each arthroscopic ICRS score group, PFAA had an accuracy of more than 85% in any group and was better that MRI. These results indicate a higher diagnostic accuracy of PFAA in all cases but was particularly better in lower degrees of cartilage damage.
For the present study, we expected a high correlation between arthroscopically confirmed patellofemoral cartilage damage and PFAA preoperative scores. We anticipated similar results as those obtained with MRI, the current non-invasive diagnostic gold standard for patellofemoral joint lesions. Previous studies have reported a high sensitivity (90%) and a low specificity (70%) for cartilage damage diagnosis with MRI, having a better accuracy in higher grades of chondromalacia (Smith et al. 2012). Our results showed an MRI specificity of 72% and sensitivity of 70%. When comparing the two diagnostic tests, PFAA had 18.32% more specificity, 13.29% more sensitivity, 9.1% better positive predictive value, and 43.62% better negative predictive value than MRI. Both the Likelihood positive and negative ratio, odds ratio and ROC area analysis favor PFAA over MRI. PFAA had a specificity of 90.33% and a sensitivity of 89.29%, including the detection of minimal or non-existent cartilage damage. The results confirmed by the weighted kappa analysis yielded favorable values of likelihood positive and negative ratios. An area under the curve of 0.97 validated a high diagnostic accuracy that was superior to MRI.
It was interesting to find that 70% of our patients with meniscal or ACL injuries had some degree of patellofemoral cartilage damage. Kobayashi et al. reported a 39% patellofemoral cartilage lesion prevalence in asymptomatic patients, and Hart et al. a 52% in patients with anterior knee pain measured by MRI (Kobayashi et al. 2016; Hart et al. 2017). In a study of 31,000 consecutive knee arthroscopies, Curl et al. reported that 63 % of patients had a chondral defect evident intraoperatively, irrespective of the surgical indication (Curl, Krome, and Gordon 1997). In a similar study of 1,000 consecutive knee arthroscopies, Hjelle et al. found that 61% of their patients had chondral or osteochondral lesions present, 19 % of which were focal (Hjelle, Solheim, and Strand 2002). While the majority of these lesions were found on the medial femoral condyle (58%), chondral lesions affecting the patella were the second most common, present in 11 % of cases. In a cohort of patients with patellar instability, Nomura et al. demonstrated that 37 of 39 patients (95%) with acute lateral patellar dislocations treated surgically were found to have chondral or osteochondral lesions affecting the patella (Nomura, Inoue, and Kobayashi 2007). Andrade et al found a prevalence of 68% of grade III-IV patellofemoral chondral defects in their 1,311 knees systematic review (Andrade et al. 2019).
Our results showed a higher overall prevalence, probably because 68.8% of our patients had ICRS 1 or 2. This means that 31.2% of our patients had ICRS 3 or 4, which correlates with the data previously described in the literature.16 Furthermore, 7.7% of our cases had full thickness cartilage lesions, a comparable finding to the 6.8% reported by Rotterud et al. (Rotterud et al. 2011). We did not find significant differences in cartilage damage or diagnostic accuracy of PFAA and MRI when stratified by gender or body weight. However, as expected, there was an increase in arthroscopically confirmed patellofemoral cartilage damage with age, and this trend was reflected in both PFAA and MRI findings (Widuchowski, Widuchowski, and Trzaska 2007; Wu et al. 2016; Wyatt et al. 2014). Notably, PFAA-ICRS scores more closely matched arthroscopic findings across all age groups, with statistically significant superiority in the 35–45 and over-65 age groups. While our findings support the potential of PFAA to provide a noninvasive, quantifiable measure of cartilage integrity, further clinical outcome studies are needed before it can be recommended for applications such as postoperative monitoring, physical therapy guidance, or population-based screening. At present, PFAA should be viewed as a promising diagnostic adjunct rather than a validated tool for preventive or wearable-based musculoskeletal assessment.
Audioarthrography has been profoundly studied in order to obtain the best possible acquisition of articular sound emissions aiming to characterize healthy from non-healthy joints (Befrui et al. 2018; Jeong et al. 2018; Kręcisz and Bączkowicz 2018; Madeleine et al. 2020; Mascaro et al. 2009; McCoy et al. 1987; Shark, Chen, and Goodacre 2011; Töreyin et al. 2016). In the present study we moved forward into the specific clinical application of audioarthrography in the patellofemoral joint. As a first step, we showed an in-vivo correlation of sound emissions with cartilage damage and were able to identify distinct patterns in different degrees of lesions confirmed by arthroscopy. We found better diagnostic accuracy than MRI in our series. We believe audioarthrography can play a role in clinical decisions when used in joints that still have diagnostic shortages, that may have cartilage damage and wear with mild or no symptoms, and where outcomes may cause major surgical procedures. The patellofemoral joint meets all of these features. PFAA offers notable advantages in cost-effectiveness when compared to conventional imaging such as MRI. The hardware is relatively low-cost, portable, and does not require contrast agents, radiation, or specialized radiological interpretation. Because it can be deployed in outpatient settings with minimal training, it may reduce reliance on more expensive diagnostic imaging—particularly for screening or longitudinal monitoring of patellofemoral cartilage health. While formal health economic analyses were beyond the scope of this study, future investigations should quantify the cost savings and workflow efficiencies associated with integrating PFAA into routine orthopedic care.
Limitations
This study is not without its limitations. First, our patient cohort consisted of individuals undergoing arthroscopy for meniscal or anterior cruciate ligament pathology. We intentionally excluded patients with primary patellofemoral conditions such as degenerative osteoarthritis or post-patellar dislocations to maintain a homogenous population. However, this introduces a degree of selection bias and may limit the generalizability of our findings to populations with isolated patellofemoral disease. The data obtained allowed us to demonstrate that PFAA provides valuable noninvasive information regarding patellofemoral cartilage damage, with correlation to arthroscopic findings that was superior to conventional MRI, a diagnostic modality which can itself be refined with special imaging programs and contrast media (Bodelle et al. 2016; Van Eck et al. 2017). Second, although we used the ICRS grading system during arthroscopy, the evaluation was not performed in a blinded fashion. While printed arthroscopic images were reviewed postoperatively for all patients, we did not capture intraoperative video for external grading. This may affect objectivity in cartilage assessment. A follow-up study is planned to incorporate video documentation and enable blinded, independent scoring by additional reviewers. Third, the interpretation of MRI findings was based on radiology narrative reports that were subsequently converted into ICRS-equivalent scores. This process introduces subjectivity and may affect the validity of comparisons. These reports were not re-analyzed by a dedicated musculoskeletal radiologist, nor was inter-reader variability assessed. A follow-up study is planned to request the reading musculoskeletal radiologist to specifically use ICRS scores in their radiologic interpretation. Fourth, clinicians interpreting both MRI and PFAA results were not formally blinded to arthroscopic outcomes. The absence of blinding may introduce interpretation bias when comparing diagnostic performance. Fifth, we did not perform a formal power analysis or sample size calculation. As this was an exploratory diagnostic performance study, future prospective trials should include powered sample designs. Sixth, we did not account for potential confounders such as synovitis, physical activity level, or comorbid osteoarthritis, which may influence joint acoustics and cartilage health. Finally, the PFAA device used in this study is investigational and has not undergone full regulatory validation. While all devices were calibrated prior to use, formal assessments of inter-device variability, cross-center reproducibility, and signal fidelity have not yet been completed. This may limit the generalizability of the findings, and future technical validation studies are warranted to confirm consistent performance across clinical environments.
CONCLUSION
In this diagnostic performance study, patellofemoral audioarthrography (PFAA) was shown to be a noninvasive, reproducible tool capable of detecting cartilage damage in the patellofemoral joint with high diagnostic accuracy. Compared to conventional MRI, PFAA demonstrated superior sensitivity, specificity, and overall concordance with arthroscopic ICRS grading across all levels of chondral injury. These findings support the utility of PFAA as a cost-effective and practical adjunct for assessing patellofemoral cartilage integrity, particularly in patients undergoing evaluation for meniscal or ligamentous knee pathology.


the_kv032_pfaa_screen_displays_demographic_data__pain_scale__laterality_and_previous_tr.png)



the_kv032_pfaa_screen_displays_demographic_data__pain_scale__laterality_and_previous_tr.png)


