Introduction
Fractures of the pelvic ring are a relatively common injury, accounting for about 2%-8% of all fractures (Grotz et al. 2005; Hu et al. 2023; Buller, Best, and Quinnan 2016; Pohlemann et al. 1996). These injuries may result from high-energy mechanism such as motor vehicle accidents or may present as insufficiency injuries in the setting of low energy mechanisms in the elderly and osteoporotic population (Hu et al. 2023; Bishop and Routt 2012). When indicated for operative stabilization, the primary goal for management of unstable pelvic ring injuries is early mobilization with minimal iatrogenic insult to the soft tissue (Bishop and Routt 2012).
Surgical techniques for stabilizing pelvic ring injuries include open reduction and internal fixation or percutaneous fixation, with an increasing trend toward the latter (Gire et al. 2018) which offers several benefits including shorter operative time, lower blood loss (Abou-Khalil et al. 2020), and lower infection risk. Despite lack of direct visualization, studies have demonstrated that percutaneous pelvic fixation achieves similar reduction quality as open procedures (Lindsay, Tornetta, Diwan, et al. 2016; Pearson et al. 2018). One of the challenges of percutaneous pelvic fixation is addressing the variability in pelvic morphology among patients (A. N. Miller and Routt 2012). Sacral dysmorphism, described in almost half the adult population (Wu et al. 2009), may complicate techniques for posterior pelvic ring fixation. For instance, the narrow and angled osseous corridors of the upper sacral segment in a dysmorphic pelvis increase the risk of cortical perforation and make a straight trajectory for a trans-sacral trans-iliac screw unfeasible (Kaiser et al. 2014).
Recent advances in surgical technique and implants have allowed for minimally invasive, percutaneous stabilization of the pelvis utilizing the concept of osseous fixation paths (Langford et al. 2013). Despite these advances, percutaneous stabilization of the pelvis continues to pose a significant challenge for surgeons due to variability in pelvic morphology (Pohlemann et al. 1996; Conflitti, Graves, and Chip Routt 2010). A novel implant aimed to alleviate this challenge is a flexible intramedullary device that once in position, can be locked rigidly (CurvaFix® Intramedullary Implant, CurvaFix, Bellevue, WA) (Zakariaee et al. 2016). In this study, we present a case series of patients at three level 1 trauma centers who underwent pelvic ring stabilization with the CurvaFix flexible intramedullary stabilization device.
Methods
Study Population and Setting
This was a retrospective case series of patients treated with flexible intramedullary fixation for pelvic ring and acetabular fractures at each of three level 1 trauma centers from 2021 to 2023. The flexible intramedullary technology used is an FDA 501k approved device for pelvic fixation. Institutional Review Board (IRB) approval was obtained for the study at each participating institution prior to data collection. Patients who underwent surgery with flexible intramedullary fixation for pelvic fractures were recorded into an internal database at each institution. Data use agreements between institutions allowed compilation of de-identified patient data for analysis. Standardized perioperative and postoperative variables were recorded.
Data Collection
All patients treated with flexible intramedullary fixation for pelvic ring and acetabular fractures between January 2021 and December 2023 were included. The data cutoff for additional patient inclusion was December 31st, 2023. Cases where flexible intramedullary fixation was aborted in favor of alternative methods of fixation were excluded. Pelvic ring fixation was occasionally performed in conjunction with other surgical procedures for additional injuries, which were also noted in the database.
Data was collected from each institution’s electronic medical records by a designated research assistant or coordinator. Variables collected for analysis included injury mechanism, high or low-energy trauma, additional orthopaedic and non-orthopaedic injuries, pelvic fracture pattern, surgical procedure(s), total operative time, estimated blood loss, any additional procedures performed in the same case, postoperative weightbearing protocol, postoperative DVT prophylaxis, length of hospitalization, postoperative complications, disposition after hospitalization (home, rehab, nursing facility), weightbearing status at follow-up, death, and cause of death. Postoperative follow up protocol was determined by the operating surgeon at each institution.
Study Definitions
Injury mechanisms were subcategorized into high energy or low energy. The latter was defined as fragility or insufficiency fractures that occurred following an event which would otherwise not be expected to result in a fracture, such as ground level falls or transfers.
Pelvic ring injury patterns consisted of sacral fractures including H or U type patterns associated with spinopelvic dissociation, pubic rami and root fractures, pubic symphyseal widening, iliac crest fractures, and sacroiliac (SI) joint injuries. The Young-Burgess classification was used to describe lateral compression, anterior-posterior compression, and vertical shear pattern injuries; however several patients presented with a combined pattern (Burgess, Eastridge, Young, et al. 1990). Weightbearing status after surgery was defined as the maximum allowed weight bearing determined by the surgeon.
Postoperative follow-up visits were recorded in weeks from the surgical date. Complications included any adverse event that occurred in the postoperative follow up period related to the surgery. Weightbearing status at follow-up was defined as the weightbearing capability of the patient at the time of follow-up regardless of postoperative weightbearing status allowed by the surgeon. For instance, if a patient was made weightbearing as tolerated (WBAT) on bilateral lower extremities postoperatively but presented to clinic with a walker, weight bearing status at follow up would be recorded as WBAT with assistive device (walker).
Statistical Analysis
Descriptive statistics were utilized to summarize the data in this case series. Sub-analyses were performed based on injury mechanism and fracture pattern. All analyses were performed by SPSS (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 29.0.2.0 Armonk, NY: IBM Corp).
Conflicts of Interest
The surgeries included in this study were performed by four surgeons at three institutions, three of which were consultants for the device manufacturer, and three who were part of the surgeon advisory board.
Surgical Technique
The following surgical technique describes placement of a trans-sacral trans-iliac flexible device at the upper sacral segment (S1).
Flexible intramedullary fixaiton was performed using the CurvaFix IM System (CurvaFix Inc, Bellevue, WA). The implants is available in 7.5mm and 9.5mm diameters, with length ranging from 90mm to 180mm.
The patient is positioned supine on a flat-top radiolucent table with a sacral bump to allow for clearance of the surgical table with instrumentation and adequate posterior access. The surgical field is prepped and widely squared off from the inferior border of the sternum to the mons pubis and inguinal folds with sterile towels followed by a sheet of iodine impregnated antimicrobial dressing (Ioban, 3M, Saint Paul, MN).
The greater trochanter and the anterior superior iliac spine (ASIS) are palpated as landmarks. A horizontal line is drawn in line with the femoral shaft, and a second line a drawn perpendicular to the first from the ASIS straight down to the table to form quadrants. Fluoroscopic imaging is used to obtain inlet and outlet views to ensure proper trajectory for the guidewire. Optionally, a line may be drawn parallel to the C-arm beam on each view to detail cephalad/caudad (outlet view) or anterior/posterior (inlet view) directionality to assist in adjusting the guidewire as necessary.
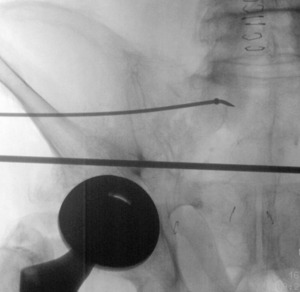
A 1.6 mm Kirschner wire (K-wire) is placed in the posterior superior quadrant down to bone. Start point is confirmed using inlet and outlet views. Once a satisfactory start point is located, the wire is tapped into the bone with a mallet. A skin incision is made, then the scalpel is run along the pin to incise fascia. A drill sleeve is placed over the pin through the incision onto bone. The K-wire is exchanged for a 2.8mm guide pin which is inserted at least 3cm into the lateral ilium, aimed toward the S1 body (Figure 1). The 3cm is necessary to match the proximal straight portion of the flexible implant. When approaching the neural foramen, a lateral image may be taken to ensure the pin is caudal to the ilio-cortical density and cephalad to the S1 tunnel to avoid injury to the L5 or S1 nerve roots.
While holding the drill sleeve in place, the 2.8mm guide pin and drill are removed and a steerable guidewire and guide sleeve are inserted into the entry hole. A T-handled chuck is fastened over the guidewire and a mallet is used to tap the guidewire into the desired trajectory, using the ball tip of the guidewire to steer an intraosseous path. The trajectory is checked frequently using inlet and outlet views to confirm the anterior/posterior and cranial/caudal location of the wire. The wire is tapped in until it crosses the opposite SI joint, noting that a fair amount of resistance is met while crossing the SI joint.
Once the guidewire is in position, a depth gauge/implant selection device (ISD) is used to measure the desired length of the flexible intramedullary implant. Next, the guide sleeve is removed while maintaining the drill sleeve. A reamer is advanced over the guidewire, stopping short of the end of the guidewire to maximize purchase in bone. The reamer is removed, and a countersink is used as needed.
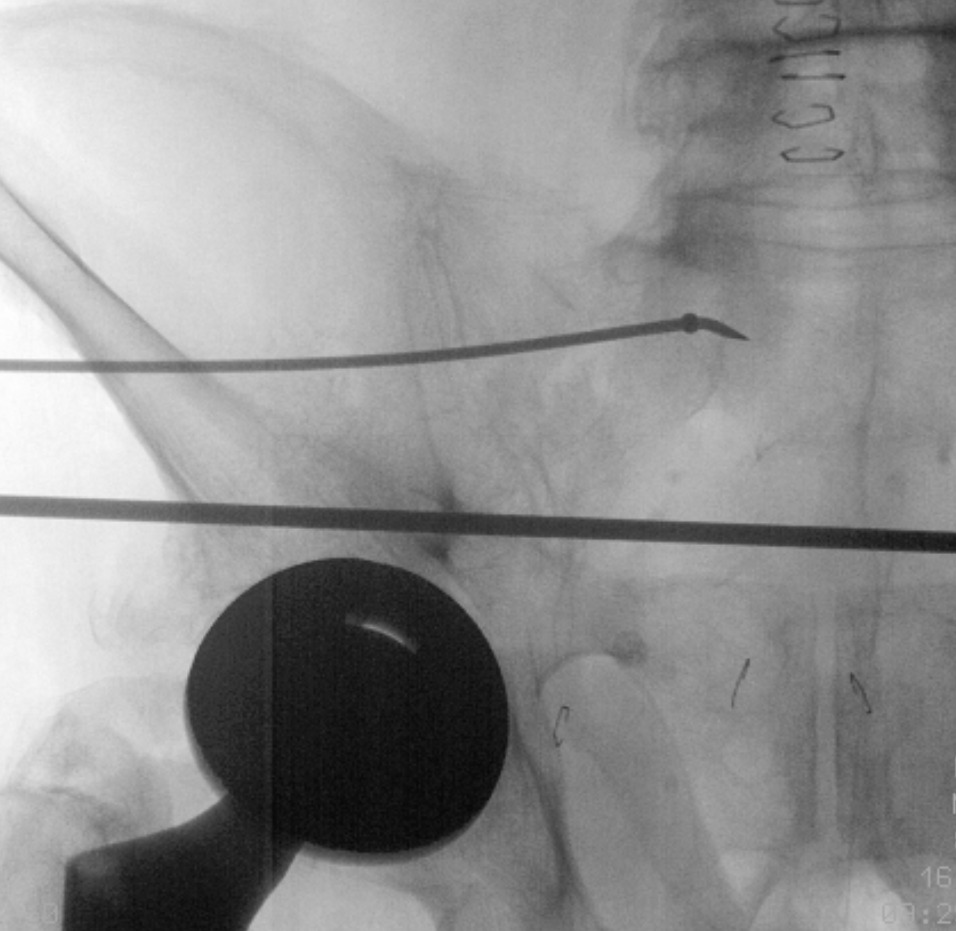
An exchange tube is placed over the steerable guidewire fully through the intraosseous channel (Figure 2). The guidewire is removed while making sure the exchange tube remains in place. A 1.6mm driving guidewire is inserted into the exchange tube. The exchange tube is then removed.
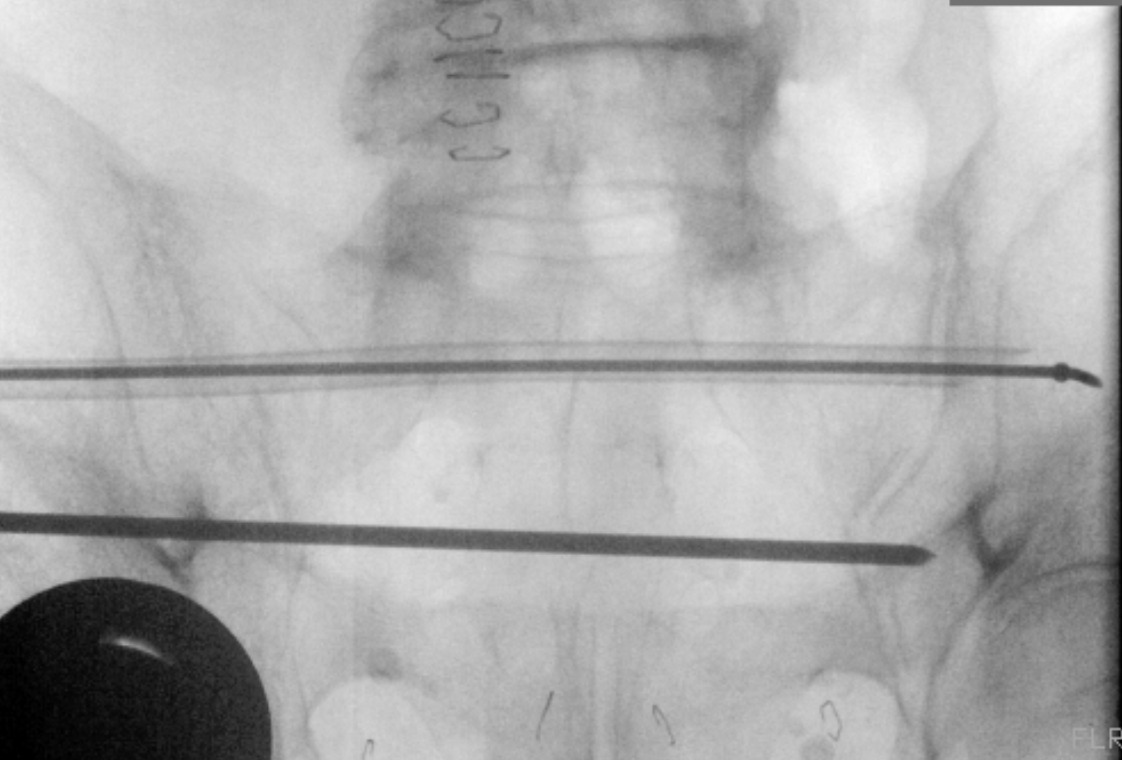
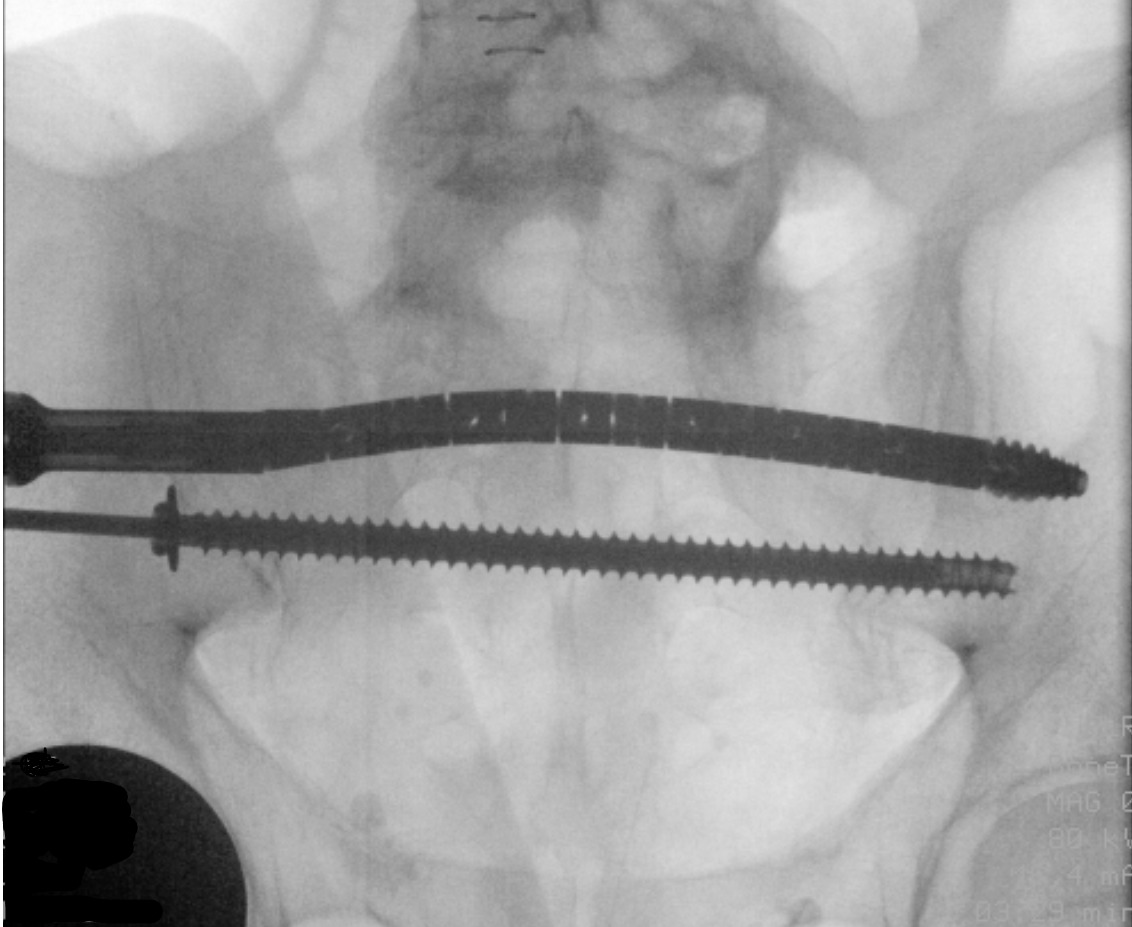
The CurvaFix implant is attached to the inserter and torque handle, then advanced over the driving guidewire using the torque handle. Once the implant is seated, the handle is turned until the inserter fins are parallel to the ground. The guidewire is removed, then the locking mechanism is activated. The inserter and torque handle are removed (Figure 3).
Results
Demographic Characteristics
From January 2021 to December 2023, one hundred and eleven patients underwent flexible intermedullary fixation for pelvic ring injuries at one of three institutions. The demographic description of patients is shown in Table 1. The mean (± SD) age of patients was 64.8 ± 20 years (range 21 to 101). 44 (39.6%) patients were male and 67 (60.4%) were female. By race, ninety patients treated with flexible intramedullary fixation identified as white or Caucasian (81.1%), nine (8.1%) identified as black or African American, and 12 declined to identify their race. Only one patient identified as Hispanic (0.9%).
Injury Mechanism, Fracture Patterns and Additional Orthopaedic and Non-Orthopaedic Injuries
Descriptions of patient injuries by mechanism and energy level of the injury, pelvic fracture pattern, and other associated injuries are detailed in Table 2. Fifty-one patients sustained pelvic ring fractures from high-energy mechanisms; of these, 30 were male (59%) and 21 were female (41%). Among the sixty patients who sustained pelvic ring fractures from a low-energy mechanism, 46 (77%) were female. When comparing energy of mechanism by sex by chi-squared testing, there was a significant difference between males and females (p<0.001) with low energy mechanisms more likely to be correlated with female sex.
The most common mechanism for pelvic ring injury in the available patient data for 59 patients was ground level fall (52.5%), followed by fall from a height such as ladders and roofs (13.5%). With regards to additional orthopaedic and non-orthopaedic injuries, 10 patients (9.0%) had a femur fracture, 10 patients had rib fractures, 4 (3.6%) had retroperitoneal hematomas, 7 (6.3%) had visceral injury to the liver, spleen or bowel, and 8 (7.2%) had pulmonary injuries including hemothorax, pneumothorax, and pulmonary contusion.
Pelvic fracture patterns are described in Table 2. Lateral compression type 1 and type 2 patterns were most common (25.2% and 22.5% respectively), and vertical shear injuries were the least common (0.9%).
Perioperative and Postoperative Outcomes
Perioperative variables including operative time and estimated blood loss, and postoperative variables such as weight bearing allowance, deep venous thrombosis (DVT) prophylaxis, length of stay, post-hospitalization discharge destination, and postoperative complications are outlined in Table 3.
The mean operative time for all cases was 101.5 minutes, but after excluding cases where part of the procedure required open dissection and reduction (including only cases with purely percutaneous fixation), the mean operative time was 89 minutes (range 22-309 minutes). The average EBL for all cases was 151 cc’s, and 122 cc’s when excluding cases requiring open dissection.
Postoperatively, ninety (81.1%) patients were made weightbearing as tolerated (WBAT), 2 (1.8%) were weight protected weight bearing with a walker, 8 (7.2%) were made toe-touch weightbearing on the operative side, and 8 (7.2%) remained non-weightbearing due to other lower extremity injuries.
Most patients (71.2%) received low molecular weight heparin (LMWH) postoperatively for deep venous thrombosis prophylaxis. 12 patients (10.8%) received aspirin, 11 (9.9%) received a direct oral anticoagulant (DOAC), and 6 (5.4%) received subcutaneous heparin. Patients at high risk for bleeding were placed on a continuous heparin drip or mechanical sequential compressive device with no chemical anticoagulation.
The mean length of stay was 11.14 days ± 9.94 days and the range of length of stay was between 0 days (discharged same day) to 68 days. Half of the patients (49.5%) were discharged to a skilled nursing facility or extended care facility after hospitalization. Thirty-eight (34.2%) patients were discharged home after inpatient physical therapy assessment. 17 (15.3%) were evaluated by a physiatrist and deemed appropriate candidates for inpatient acute rehabilitation facilities. One patient expired during hospitalization.
Complications
Nine patients (8.1%) had documented complications intraoperatively and postoperatively related to pelvic fixation with a flexible intraosseous fixation, and five patients (4.5%) had medical complications during their hospitalization, detailed in Table 4.
Intraoperative complications included two cases of mal-positioned implants, one of which returned to the OR for revision. There was one case of bladder rupture which required urology consultation and repair intraoperatively. Lastly, there was one case of implant failure where the implant broke during placement into the pelvis, with inadvertent fracture of the iliac bone while retrieving the broken fragment. Postoperative complications included one case of sacral nonunion requiring revision, two nerve palsies, one wound dehiscence with superficial surgical site infection, and two cases of osteomyelitis.
Five patients had documented postoperative medical complications, which included need for pressors for postoperative hypotension, GI bleed, two cases of ileus, pulmonary embolism, and death from upper airway obstruction.
Follow-up Weightbearing Status
Follow-up data ranged from 10 to 123 weeks (3 to 28 months) postoperatively. The patient’s functional weightbearing ability at the most recent follow up was recorded (Table 5). Nine patients (8.1%) did not have any outpatient follow-up data available.
Excluding patients without follow-up, fifty-one (50%) patients were independent ambulators without assistive devices at their last outpatient follow-up appointment. Eleven patients (10.8%) were using a cane, and 29 (28.4%) were weight-bearing with a walker or rollator. Eight patients remained non-ambulatory or wheelchair bound, one due to unilateral lower extremity amputation, and another due to baseline dementia.
Discussion
This multicenter case series describes the early experience on 111 patients who underwent a novel flexible intraosseous fixation technique for pelvic ring injuries. This is the largest cases series reported to date that follows patients who received flexible fixation. While there have been several case reports of successful surgeries using this new implant (H. S. Miller and Gardner 2022), there is a dearth of information regarding postoperative complications and early functional outcomes.
All patients that were treated consecutively with this implant between January of 2021 and December of 2023 at three level 1 trauma centers were included, and early perioperative and postoperative outcomes were reported. Overall, there were few complications related to the surgery and excellent return to function postoperatively at the latest follow-up visit. Specifically, there was a 3.6% rate of intraoperative complications (4 patients) and 4.5% rate of postoperative complications (5 patients) related to the surgical procedure in our series, totaling less than 10% chance of any complications related to flexible intraosseous nailing. A third (34.2%) of the patients treated were discharged home in good condition after their hospitalization. One-half (50.0%) of the patients who followed postoperatively were able to return to weightbearing without assistive devices during their early postoperative follow up period.
Previous studies have shown that early weightbearing after pelvic ring fixation is safe, with no difference in complications such as implant failure, malunion, or loss of reduction compared to delayed weightbearing protocols (Marchand, Working, Rane, et al. 2019). Percutaneous fixation has the added benefit of reduced pain and narcotic use compared to open fixation (Benhenneda et al. 2022). In our series, 81% of patients were made fully weightbearing as tolerated immediately postoperatively, with no reported failure of hardware or fixation. The majority of patients who were limited from full weightbearing postoperatively despite stable pelvic fixation had additional lower extremity injuries which may have restricted weightbearing. Early physical therapy and rehabilitation after pelvic fixation may prevent additional complications such as venous thrombosis, skin ulcers, muscle atrophy and deconditioning, similar to geriatric hip fractures (Zuckerman 1996; Wendt, Heim, Josten, et al. 2016).
The most common pelvic ring injuries requiring fixation in this study were lateral compression 1 (LC1) injuries. There is currently no clear consensus on the management of LC1 injuries, however up to 37% of LC1 injuries have been shown to be unstable with an exam under anesthesia, which highlights the role of surgical fixation, which may improve time to mobilization, reduce complications associated with prolonged immobility, and improve long-term functional outcomes (Sagi, Coniglione, and Stanford 2011; Varma, Foxall-Smith, Donovan, et al. 2022). Surgeons have used inability to mobilize due to pain as an indication for surgical intervention (Rommens, Boudissa, Krämer, et al. 2021). A multi-center study by Tornetta et al. demonstrated higher pain scores in patients treated nonoperatively for lateral compression injuries at 3 months, although the difference between the nonoperatively and operatively treated groups was small (Tornetta, Lowe, Agel, et al. 2019).
Complications related to surgery in our study included two cases of mal-positioned implants and one bladder rupture. The latter is a rare complication related to pelvic surgery that has previously been reported in literature (Stenquist, Chavez, and Weaver 2019). Appropriate fluoroscopic visualization is necessary for any procedures requiring percutaneous fixation strategies and may be affected by patient factors such as bowel gas, osteoporosis, or excessive body mass index. Traditional percutaneous screw fixation may be especially difficult, if not impossible, in certain patients with sacral dysmorphism where standard approaches are not feasible. In conventional sacroiliac screw fixation, the incidence of screw malposition is 3-25% and the rate of neurologic damage is as high as 18% (Zarei et al. 2022). Only 2 of 111 (1.8%) patients had mal-positioned implants in our series, and 2 patients had documented nerve palsies following surgery. The flexible nature of the implant allows for curvilinear passage through a dysmorphic sacrum, which may account for the lower rate of implant malposition in our series. There was one report of a broken implant in our series, resulting in additional surgical time for implant retrieval and iatrogenic fracture of the iliac wing in the patient. Despite this being a new procedure with a learning curve for the surgeon, the complication rate in our study is lower than traditional percutaneous screw fixation.
Our study has several limitations. First, the study describes early outcomes for a series of patients who underwent a relatively novel technique of pelvic fixation, and therefore follow-up periods were limited to less than 3 years. Future follow-up is necessary to assess long-term outcomes after pelvic fixation. Additional limitations exist due to the retrospective nature of the study - the three institutions participating in this study did not have a standardized follow up protocol, limiting our ability to compare patients’ functional capacities at specific time periods postoperatively. Specific patient reported outcome measures were not available for analysis. Baseline ambulatory status was unknown for several of the patients included in the study, which may bias outcomes to poorer results in ambulatory status if not accounting for patients who were minimally ambulatory at baseline. The complications discussed here were taken from documented physician and nursing notes in patient record; however, reporting bias may be present if certain complications were not specifically documented. In particular, bladder injury and femoral or sciatic nerve palsies may have been related to the initial injury or perioperative traction, and it is unclear whether these complications were directly related to surgery. Given that we have short-to-mid-term follow up data available on this patient population, potential complications related to painful hardware requiring removal may not be apparent in this population at this time. Lastly, although this is the largest case series of patients treated with a flexible intraosseous implant currently, the information provided is purely observational, and a significantly larger number of cases is needed to make meaningful statistical analyses of outcomes after surgery. As with all novel technologies, early adopters should take significant precautions when deciding to use this implant and careful patient selection is critical.
Our case series demonstrates that flexible intraosseous fixation may be a safe, novel technique to treat various types of pelvic ring injuries, especially when helping navigate intraosseous pathways in patients with dysmorphic sacra. Our early experience at three institutions demonstrates a low complication rate comparable to known complications rates of standard percutaneous pelvic fixation. Additional future directions may look at complications rates of standard screw fixation compared to flexible fixation specifically in patients with sacral dysmorphism. Our study provides positive data to support the safe use of this novel device. Longer-term follow up data and more cases are needed to confirm our early positive outcomes with flexible intraosseous fixation of pelvic ring and acetabular injuries.