Introduction
In years prior to widespread tranexamic acid usage, major elective orthopedic surgeries, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA), often involved significant blood loss, with 37% of THA and 25% of TKA patients requiring blood transfusions for postoperative anemia (Blumberg, Kirkley, and Heal 1996). Blood loss during THA averaged 1000–2000 mL, while TKA resulted in losses of up to 2000 mL (Kim, Park, and Davey 2015; Toy et al. 1992). To mitigate this, tranexamic acid (TXA), an antifibrinolytic lysine analog, has become a widely adopted pharmacological strategy.
Tranexamic acid (TXA) promotes hemostasis by inhibiting plasminogen activation and preventing plasmin-mediated fibrin degradation (Haratian et al. 2021). Its efficacy was first demonstrated in the CRASH-2 trial, which evaluated TXA in over 20,000 trauma patients, showing significant reductions in mortality related to hemorrhage. Similarly, the MATTER study in combat trauma reinforced the benefits of TXA in reducing mortality, particularly in patients requiring extensive transfusions (Mula, Parikh, and Suresh 2020). These findings spurred interest in the application of TXA within orthopedic surgical practice.
Tranexamic acid can be administered intravenously (IV), topically, or intra-articularly (IA) (Haratian et al. 2021). Dosing regimens vary between bolus, continuous infusion, single, or multiple doses, and may be delivered preoperatively, intraoperatively, or postoperatively. The flexibility in administration has allowed for its integration into various clinical protocols, especially in joint arthroplasty.
Total hip arthroplasty and total knee arthroplasty are associated with substantial blood loss, impacting hospital stays, transfusion needs, and patient recovery. TXA use reduces the dependence on costly allogeneic transfusions, which carry risks of reactions and infections (Haratian et al. 2021; Kalairajah et al. 2005). Meta-analyses, such as the one by Zhao et al., indicate that IV TXA reduces transfusion rates effectively, while topical TXA minimizes total blood loss. Oral TXA has also demonstrated efficacy, showing comparable benefits in blood loss reduction and reduced thromboembolic risks compared to no TXA use (Toy et al. 1992; Wang et al. 2019; Zhao, Ma, and Ma 2019).
Perioperative TXA is now a standard of care in total joint arthroplasty (TJA). Its use in TKA has been linked to reduced blood loss, transfusion rates, early swelling, and ecchymosis, all of which contribute to faster recovery and safer outpatient procedures (Kirwan et al. 2024). Concurrent administration of TXA and aspirin postoperatively in TKA patients has also been linked to decreased joint stiffness and accelerated functional recovery (Frederick et al. 2023).
While the role of TXA in hemostasis is well-established, its potential as a deep vein thrombosis (DVT) promoter remains a concern. The current study aims to answer the question: does extended-length TXA lead to a higher risk of DVT as compared to day-of-surgery-only TXA administration patients?
Methods
Ethical Approval Statement
This study was reviewed and approved by the Mohawk Valley Health System, Inc. Institutional Review Board at Wynn Hospital (Approval Number: 2024-FB-008). All procedures performed in this study complied with the ethical standards of the institution and the Declaration of Helsinki. Informed consent was obtained from all participants included in the study.
Study Design and Participants
This retrospective, single-surgeon cohort study was conducted at two distinct clinical sites: an outpatient surgery center and a hospital setting for in-patient care. Data were collected from patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA) between May 2023 and November 2024. All patients not on prescription anticoagulation were instructed to take 81 mg aspirin two times a day post-operatively for DVT prophylaxis. All patients were also prescribed tranexamic acid 1300 mg orally daily for 15 days postoperatively. Exclusion criteria included patients on chronic anticoagulation therapy for other medical comorbidities.
A total of 576 patients undergoing TKA and 381 patients undergoing THA were included in the study. All patients were treated in accordance with standardized perioperative protocols, and follow-up was conducted for a period of 6 weeks postoperatively.
Intervention Protocol
All patients in the study were initiated on a pharmacological regimen consisting of 81 mg of aspirin (ASA) administered twice daily for 4 weeks. In addition, each patient received 1300 mg of tranexamic acid (TXA) orally daily for 15 days. This regimen was designed to reduce postoperative bleeding. Additionally, all participants were prescribed a standardized home physical therapy protocol that focuses on swelling prevention to promote faster functional recovery and minimize complications, including DVT. The home physical therapy was divided into two stages, weeks 1 through 2 and weeks 3 through 6. This protocol was followed as described by Wickline et al. in 2023 and weeks 1 through 2 focused on the use of a low-sodium anti-inflammatory diet, micronutrient supplementation to diet such as amino acids and micronized purified flavonoid fraction (MPFF), easy-to-use compression devices, limited weight-bearing exercises with the use of assistive device for 1 week, 5-8 minutes of ROM exercises every hour, limb elevation, applying ice to the surgical site, and step count. Weeks 3 through 6 focused on the use of MPFF and micronutrient supplementation to diet, compression devices, ROM exercises, application of ice to the surgical site, and step count (Wickline et al. 2023).
Outcome Measures
The primary outcome of the study was the incidence of symptomatic deep vein thrombosis (DVT), which was evaluated clinically. Symptomatic DVT was defined by the presentation of clinical symptoms including swelling, pain, redness, and/or positive imaging results (e.g., ultrasound) consistent with DVT. The incidence of symptomatic DVT in both THA and TKA cohorts was compared to the rates reported in the literature by Bala et al. 2017 and Warren et al. 2020 to determine if the intervention protocol affected DVT incidence relative to standard practices.
Secondary Outcome Measures
Secondary outcomes included the evaluation of knee range of motion (ROM) following TKA. Range of motion measurements were obtained at two time points: 2 weeks and 6 weeks postoperatively. These measurements were recorded using a standard goniometer, and changes in ROM over the follow-up period were analyzed to assess functional recovery.
Statistical Analysis
The rates of symptomatic DVT were compared between groups using chi-square tests for categorical data, with significance set at p < 0.05. Descriptive statistics were used to summarize clinical outcomes. All data were analyzed using SPSS version 28.0 (SPSS Inc., Chicago, IL) for Windows.
Results
A total of 957 patients were included in this retrospective study, with 576 patients undergoing total knee arthroplasty (TKA) and 381 patients undergoing total hip arthroplasty (THA). The mean age of the TKA cohort was 68.6 ± 7.8 years, and for the THA cohort, it was 66.5 ± 8.4 years. Baseline comorbidities, including diabetes mellitus, hypertension, and obesity, were similar between the two groups, and there were no significant differences in preoperative anticoagulation use.
Incidence of Symptomatic DVT
The primary outcome of symptomatic deep vein thrombosis (DVT) incidence was assessed at 6 weeks. In the TKA group, the overall incidence of symptomatic DVT was 0.87% (n = 5) at the 6-week follow-up. The DVT rates for the TKA group using the 81 mg aspirin twice a day along with 1300 mg TXA orally daily postoperatively was 0.87% at 6 weeks. In the THA group, there were no recorded DVT.
In the TKA group, there were 5 patients diagnosed with DVT post-operatively. Table 1 summarizes the location of the DVT, the age of the patient, and the postoperative day that the DVT was diagnosed for the TKA group. THA group had no incidence of DVT.
Range of Motion Following TKA
Secondary outcomes included the evaluation of knee range of motion (ROM) in the total knee arthroplasty (TKA) cohort at 2 and 6 weeks postoperatively. At the 2-week mark, the mean knee ROM was 110.52° ± 12.4°, improving to 120.2° ± 7.9° by 6 weeks. The surgeon’s inclination is to aggressively pursue manipulation if flexion is <105° at 30 days post-op. Of the 576 patients included in the study, 16 required manipulations under anesthesia (2.7%). Of the patients that required manipulation under anesthesia, manipulation was performed at an average of 5.5 weeks after TKA.
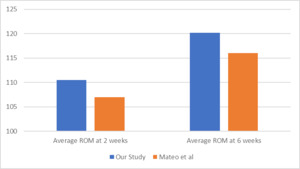
Figure 1 presents a comparison of the average ROM at 2 and 6 weeks postoperatively for TKA patients in this study, alongside the reported averages for patients not receiving extended tranexamic acid (TXA) in the study by Kirwan et al. Additionally, it includes ROM data from a retrospective study by Kittelson et al., which aimed to establish a reference chart for ROM post-TKA by recording ROM in 1,173 TKA patients.
Figure 2 compares the ROM outcomes at 2 and 6 weeks postoperatively for TKA patients in this study with those reported for patients receiving extended TXA in the study by Kirwan et al.
Adverse Events and Complications
Adverse events related to the TXA intervention were minimal. In the TKA group, one patient (0.2%) reported a skin rash which the patient attributed to TXA usage, which resolved upon discontinuation of the medication. Postoperative neurological and cardiovascular complications observed in this study included transient ischemic attack (TIA), stroke, posterior reversible encephalopathy syndrome (PRES), and cardiopulmonary arrest. TIA and stroke were noted as significant complications following total knee arthroplasty (TKA), though only one patient had a TIA and one patient had a stroke. PRES, a rare but serious neurological disorder characterized by reversible vasogenic edema, was identified in a patient following total hip arthroplasty (THA). Additionally, one female patient in the THA group experienced cardiopulmonary arrest; however, a CT angiogram revealed no evidence of pulmonary embolism. The incidence of major complications in this study for TKA group was 0.35% and that in THA group was 0.52%.
Discussion
The findings of this study indicate that the rate of symptomatic DVT was 0% for THA and 0.87% for TKA. Bala et al. (2017) reported symptomatic DVT rates in post-TKA patients as follows: 2.9% for those receiving factor Xa inhibitors, 3% for those on aspirin, 3.5% for those on enoxaparin, and 4.8% for those on warfarin. Warren et al. (2020) reported the incidence of DVT post-THA as 0.4% and the incidence of DVT post-TKA as 0.9%. Additionally, Simon et al. (2023) analyzed a cohort of TKA and THA patients receiving enoxaparin, apixaban, aspirin, warfarin, or rivaroxaban, reporting a combined symptomatic DVT rate of 1.19%. In terms of major complications, this study found an incidence of 0.35% in the TKA group and 0.52% in the THA group—both notably lower than the complication rates reported by Heo et al. (2020), which were 14.4% for TKA and 9.5% for THA, and those reported by Curlewis et al. (2023) at 6.9% for TKA and 5.1% for THA. Additionally, the THA complication rate in this study is substantially lower than the 27.32% reported by Patel, Nham, Zalikha, et al. (2023).
The secondary outcome of knee ROM following TKA at 2 and 6 weeks also demonstrated positive functional recovery in patients receiving extended TXA. These findings align with data from the AAHKS research study by Kirwan et al., further supporting the benefits of prolonged TXA use. Importantly, TXA does not appear to act as a DVT initiator, reinforcing its safety profile in joint arthroplasty patients.
As the secondary outcome measurement of this study shows better ROM at both 2 weeks and 6 weeks, it could be beneficial for patients to be started on a regimen of TXA and ASA to allow for better functional recovery without the risk of increased DVT incidence. A study done by Kittelson et al. in 2020 looking at 1173 patients without extended length TXA, showed that the average ROM post TKA in patients at 2 weeks was 97 degrees and at 6 weeks was 110 degrees. Compared to Kittelson, our study showed increased ROM at both 2 weeks (110.5°) and 6 weeks (120.2°). The ROM results reported in this study are comparable to the data reported by Kirwan et al. in 2024 which recorded ROM data for patients on an extended course of TXA.
The presumed explanation for the improved functional recovery in TKA patients is a decrease in recurrent bleeding within the joint during flexion maneuvers due to the daily inhibition of fibrinolysis.
Despite these promising findings, the study has several limitations. This study is a single surgeon series with a currently unconventional post-op protocol (no formal therapy, no tourniquet, limited step counts, etc.) and may not be generalizable to hip and knee arthroplasty patients in other practices. The 6-week follow-up period may not capture late-onset thromboembolic events, limiting long-term safety assessments. Additionally, the retrospective design introduces potential selection bias, and the absence of a direct control group makes it difficult to isolate the effects of TXA from other variables when looking at the secondary outcomes of accelerated ROM recovery.
Conclusion
This study demonstrates that extended-length tranexamic acid (TXA) in patients undergoing total knee arthroplasty (TKA) and total hip arthroplasty (THA) does not increase the incidence of symptomatic deep vein thrombosis (DVT) when compared to standard TXA administration protocols.
Although the primary focus of the study was to determine DVT rates in patients receiving extended-length TXA, the secondary outcome of accelerated TKA range of motion (ROM) recovery is promising for earlier restoration of normal activities of daily living.