INTRODUCTION
Anterior cruciate ligament (ACL) tears are a common orthopedic injury, affecting more than 200,000 individuals each year in the United States (Musahl and Karlsson 2019). An estimated 175,000 ACL reconstruction (ACLR) procedures are performed yearly, amounting to $7 billion in healthcare costs (Musahl and Karlsson 2019; Synovec, Shaw, Antosh, et al. 2019). Furthermore, the incidence of these injuries continues to increase over time (Sanders, Maradit Kremers, Bryan, et al. 2016). Although clinical and functional outcomes post-ACLR are generally positive, 35-45% of athletes who undergo ACLR are unable to return to their pre-injury levels of activity (Salem et al. 2019). Additionally, certain risk factors can increase the chances of an ACL rupture, including sex (3x greater risk in females compared to males), age (greater risk in people 18 years or younger), and participation in sports (Musahl and Karlsson 2019; Kaeding, Léger-St-Jean, and Magnussen 2017). Sex and age are also risk factors for ACL re-rupture after primary ACLR; females are at greater risk of re-rupture than males, as are younger patients (Salem et al. 2019).
ACLR is performed using autografts or allografts. Autografts are typically preferred due to their superior results and lower failure rates to date (Chalatsis, Mitrousias, Siouras, et al. 2023). Advantages to using allograft include reduced donor site morbidity, graft availability, decreased surgical time, and smaller surgical incisions. However, disadvantages to using allograft include increased risk of infection or immunogenic reaction, increased laxity over time, and increased risk of failure, particularly in young athletes (Musahl and Karlsson 2019; Dhillon et al. 2022). Studies have also demonstrated that allografts tend to incorporate and revascularize at a slower rate post-implantation compared to autografts (Iosifidis and Tsarouhas 2010; Muramatsu, Hachiya, and Izawa 2008). This can be an important factor to consider when determining rehabilitation protocol and time to return to play post-operatively (Iosifidis and Tsarouhas 2010).
To improve consistency of clinical outcomes and facilitate graft incorporation in ACLR, various graft augmentation techniques have been developed and implemented. Historically, synthetic devices were used to replace the graft altogether, yet most of these devices are no longer in use due to reported failures and complications (Batty et al. 2015). More recently, a nonabsorbable suture tape has been used to augment grafts in ACLRs (Parkes, Leland, Levy, et al. 2021a). However, the suture tape is a synthetic device that only provides mechanical reinforcement. Additionally, suture tape has the potential to stress shield the graft due to its high stiffness, which could lead to long-term graft weakness (Viens et al. 2014; Kulwin et al. 2021). In one study comparing patients who underwent hamstring autograft ACLR with vs. without suture tape reinforcement, 14% of patients in both groups underwent reoperation. Graft failure occurred in one patient in the suture tape group and four patients in the control group. IKDC, Lysholm, and return-to-sport rates were not significantly different between the two groups at final follow-up (Parkes, Leland, Levy, et al. 2021b). In a meta-analysis of all ACLR studies where the graft was augmented with suture tape, it was found that adding suture tape increased strength, stiffness, and cyclic displacement of the graft construct when compared to standard ACLR. However, this did not lead to a significant difference in retear rate clinically (Raja et al. 2023).
The goal of augmenting ACL grafts is to provide both mechanical and biological support during the early stages of graft healing. Additionally, hamstring autografts may be augmented if, upon harvesting, the graft diameter is determined to be suboptimal: smaller hamstring autografts (less than 8mm in diameter) have been shown to be predictors of poor functional outcomes and may increase the risk of ACL revision surgery (Mariscalco, Flanigan, Mitchell, et al. 2013). Therefore, surgeons may look to augment smaller grafts to increase graft diameter, thus improving patient outcomes.
In 2021, a reinforced bioinductive scaffold, BioBrace® (CONMED, New Haven, CT), was cleared for use by the US Food and Drug Administration for “reinforcement of soft tissue where weakness exists”. It is composed of a highly porous type I collagen sponge reinforced with bioresorbable poly (L-Lactide) (PLLA) microfilaments. The scaffold has been shown to encourage the induction, maturation, and remodeling of host tissue and load shares at time zero of implantation, providing both strength and biology to the repair (McMillan, Arciero, and Ford 2021; Carter et al. 2021). Its open 3D architecture supports the formation of new native tissue in and around the implant as early as 3 weeks post-implantation. It is an off-the-shelf solution that can add 1-2mm to the diameter of a graft post-harvest. This case report presents the use of BioBrace to augment an autologous hamstring graft in an ACL reconstruction.
CASE REPORT
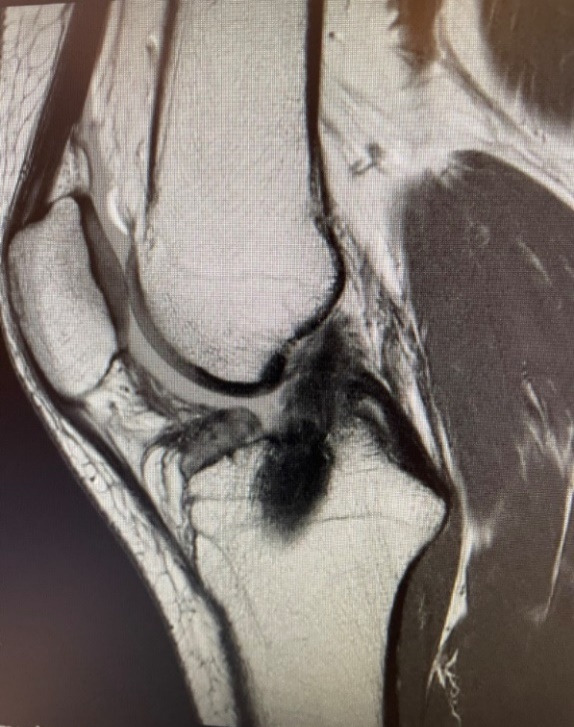
A 25-year-old female fell while skiing and suffered a twisting injury to her right knee. MRI confirmed a complete rupture of her ACL (Figure 1), as indicated by the high intensity signal at the site of injury. She was scheduled to undergo ACL reconstruction. The patient, an avid soccer player as well as a high-level skier, works with children and kneels often in her daily life; she expressed concern that a BTB graft may lead to anterior knee pain post-operatively. As a result, the decision was made to use a hamstring autograft. Part of the pre-operative discussion centered around the fact that hamstring autograft size is highly variable. Thus, it was determined that augmenting the hamstring graft with a 5 x 250 mm BioBrace implant was a viable option if the graft was undersized upon harvest.
A 4-strand hamstring graft (gracilis and semitendinosus) was harvested and measured at 7.5 mm. A Roman sandal suture technique was used to incorporate BioBrace alongside the quadruple-stranded hamstring graft. Modified Krakow sutures were placed on the free ends of BioBrace and each of the strands of the hamstring tendons. With BioBrace, the augmented graft diameter was measured at 9 mm (Figure 2). A circumferential suture was placed 25 mm from the apex of the construct, to ease graft passage during implantation. In addition to adjustable suspensory fixation on the femoral side, a 9 mm screw with sheath was used for tibial fixation (Figure 3).
Post-operatively, the patient was placed in a range of motion brace. Physical therapy was initiated three days after surgery. Bracing was discontinued at 6 weeks post-operation, and the patient was allowed to increase her activity level based on benchmarks she achieved during physical therapy.
The patient was able to resume activities involving cutting motions at 6 months post-operation and continues to be an avid runner, frequently running 10k races. At 18 months post-operation, she reported that she continues to feel no pain, runs four to five times a week, and does strengthening exercises such as leg presses regularly. She demonstrated her agility post-operatively by performing a box jump and double leg hop (Video 1). Furthermore, the physical exam conducted at 18 months post-operation revealed equal range of motion to her contralateral knee, a negative Lachman test, a negative anterior draw sign, a negative pivot shift test, and quadriceps tone equal to her contralateral knee (Video 2). She reported a SANE score of 100, demonstrating a fully functional joint with no limitations or pain.
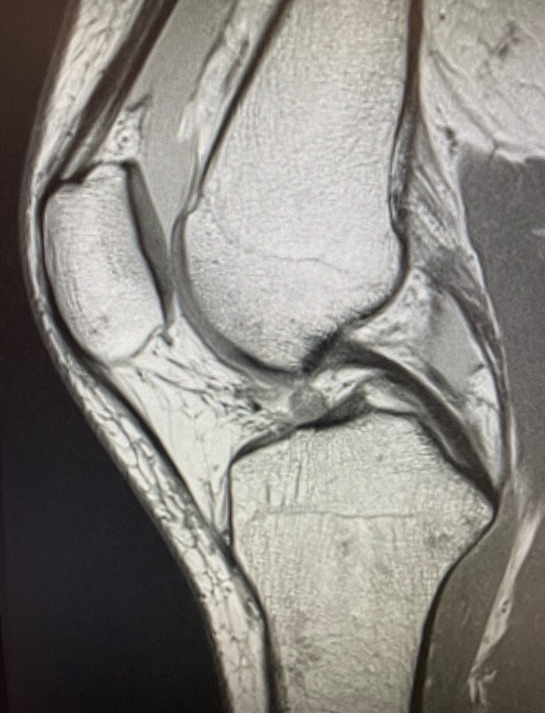
As part of her post-operative evaluation, a follow-up MRI was performed 18 months after surgery. This MRI demonstrated a robust, well-healed ACL graft (Figure 4), characterized by homogeneous low intensity signals throughout the graft construct. This corresponds to Grade I on the MRI grading scale proposed by Howell et al (Howell, Clark, and Blasier 1991)., which is indicative of a mature, well-integrated graft.
DISCUSSION
A partial or complete tear of the anterior cruciate ligament is a common injury in athletes, particularly in those who play sports that involve cutting and pivoting motions. ACL tears are typically treated with reconstruction or repair of the torn ligament. The goal of both ACL reconstruction and repair is to restore knee stability and to allow the patient to return to full activity. However, the procedures differ in their surgical approach and methods: in a reconstruction, the ACL is replaced with a tissue graft, whereas in a repair, the native ACL tissue is reattached to the insertion point. Both ACL repairs and reconstructions may be augmented based on the patient’s needs. Despite growing data on ACL repair, ACL reconstruction remains the standard of care for many. ACLR typically leads to lower failure rates and increased knee stability. Furthermore, only select tear patterns can be repaired (Hughes et al. 2021).
The three most common graft options in ACLR are bone-patellar tendon-bone (BTB) graft, hamstring tendon graft, and quadriceps tendon graft. The optimal graft for ACL reconstruction remains contested, and grafts are usually chosen on a case-by-case basis. Studies have shown no significant difference in failure rates when comparing BTB, hamstring, and quadriceps grafts in ACL reconstruction (Salem et al. 2019; Ashy, Bailey, Hutchinson, et al. 2023; Haybäck, Raas, and Rosenberger 2022). While BTB grafts are favored due to their strength and stiffness and potential for bone-to-bone healing in the tunnels, they may also be associated with anterior knee pain post-operatively and may require a larger incision to harvest the graft (DeFazio, Curry, Gustin, et al. 2020; Miller and Gladstone 2002). One study reported anterior knee pain in 52% of patients who underwent ACLR with a BTB graft (Widner, Dunleavy, and Lynch 2019). Hamstring grafts may be favored because they are associated with less harvest site morbidity and less pain post-operatively (Salem et al. 2019; Miller and Gladstone 2002). However, hamstring autografts have been associated with hamstring or knee flexion weakness post-operatively and have also been shown to stretch over time, which could potentially affect rotational stability (DeFazio, Curry, Gustin, et al. 2020). While recent data has demonstrated that quadriceps grafts are a viable option for ACLR with comparable outcomes to the other two graft options, long-term data is still needed to verify their effectiveness (DeFazio, Curry, Gustin, et al. 2020).
One of the concerns with a hamstring autograft is variability of size upon harvesting the graft. Therefore, the ability to augment the graft to increase graft diameter is an attractive option. Compared to other augmentation devices in the market, BioBrace is unique: it provides load-sharing strength to the graft at time zero and supports biologic healing, promoting graft integration, maturation, and remodeling before completely resorbing.
For this patient, we chose to augment the hamstring graft with BioBrace to increase graft diameter and initial strength. Female athletes, who are at greater risk of ACL re-rupture, may benefit significantly from graft augmentation to promote strength and facilitate early incorporation, potentially improving clinical outcomes. The MRI captured at 18 months post-operation depicts a robust, well-healed ACL graft, suggesting that BioBrace had fully incorporated into the remodeled graft.
Potential disadvantages of augmentation of ACLR with BioBrace include increased cost and added surgical complexity. Future studies on augmentation of ACLR with BioBrace are needed to validate the clinical impressions described in this case report. Larger cohort studies evaluating graft failure rates, re-injury risk, and return-to-sport outcomes at 2-, 5-, and 10-year intervals would strengthen the current findings. Additionally, longitudinal imaging (e.g., MRI or CT) could provide insight into the extent of graft integration facilitated by BioBrace. Finally, as the use of BioBrace with hamstring grafts continues to expand, comparative studies across graft types will be essential to determine optimal surgical strategies.
CONCLUSION
This is a promising example of a case in which BioBrace augmentation of a tissue graft in an ACLR setting led to an excellent patient outcome. Although the use of BioBrace for ACLR augmentation has been previously documented in the literature (Benson, Pyrz, Wood, et al. 2024; Serour et al. 2024; Pyrz, Wood, Campbell, et al. 2023; Bi, Hughes, Savage-Elliott, et al. 2024), this case study is the first to describe the use of BioBrace when augmenting a hamstring graft. The ease of incorporating BioBrace with a hamstring autograft allowed for a larger, stronger graft and, consequently, an accelerated rehabilitation protocol. These results suggest that BioBrace augmentation of a hamstring graft for ACLR can lead to outstanding outcomes in an athletic patient population with a historically high rate of failure.


__hamstring_aut.png)
.png)

__hamstring_aut.png)
.png)