Introduction
Total knee arthroplasty (TKA) is one of the most effective surgical treatments for advanced knee osteoarthritis, offering significant pain relief and improved mobility. However, managing postoperative pain remains a persistent challenge for this procedure. Inadequate pain control can slow recovery, prolong rehabilitation, and increase reliance on opioid medications (Gan 2017). While opioids have long been the standard for post-TKA pain management, their use comes with well-documented risks, including respiratory depression, gastrointestinal complications, prolonged hospital stays, and, in some cases, opioid dependence (Trasolini, McKnight, and Dorr 2018). Alarmingly, studies show that 10% to 82% of TKA patients continue opioid use beyond the immediate postoperative period, with some developing long-term dependency (Namba et al. 2018; Hadlandsmyth et al. 2018; Cancienne et al. 2018). Given these risks, there is a growing push toward multimodal pain management strategies—approaches that integrate different techniques to reduce opioid reliance while ensuring effective pain control (Memtsoudis et al. 2018; Parvizi and Bloomfield 2013).
One hopefull technique gaining attention is cryo-neurolysis, which offers a non-opioid solution for managing post-surgical pain. This approach uses extremely cold temperatures to temporarily disrupt nerve function, effectively blocking pain signals while preserving the nerve’s structural integrity (Trescot 2003). The science behind cryo-neurolysis is well established: by lowering nerve temperature to between −60°C and −80°C, the process induces Wallerian degeneration, where the axon temporarily loses function but the myelin sheath remains intact, allowing natural regeneration without permanent damage(Lloyd, Barnard, and Glynn 1976; Onik et al. 1988). The concept of using cold for pain relief dates back to ancient Egyptian and Greek medicine, but modern cryo-neurolysis emerged in the 1960s with the development of automated cryosurgical devices (Dasa et al. 2014). Over time, its use expanded from chronic pain management to surgical pain control, particularly in orthopedic procedures (Ilfeld, Preciado, and Trescot 2016).
Cryo-neurolysis in total knee arthroplasty (TKA) has primarily been studied as a preoperative intervention, typically applied 1–2 weeks before surgery to target the superficial genicular nerves and provide extended postoperative pain relief (Dasa et al. 2016; W. M. Mihalko et al. 2019). Clinical trials have shown that patients receiving preoperative cryo-neurolysis require fewer opioids and experience reduced pain for up to 12 weeks postoperatively, which demonstrates its effectiveness as a pain management strategy (Ilfeld, Gabriel, and Trescot 2017). In contrast, intraoperative cryo-neurolysis has been effectively utilized in thoracic surgery for intraoperative pain management, but its potential benefits in TKA remain largely unexplored. By applying cryo-neurolysis intraoperatively in this study, we aimed to combine the advantages of both approaches—enhancing precision, optimizing nerve targeting, and potentially improving long-term analgesic outcomes.
Despite its advantages, several important questions still remain unanswered. While studies confirm the general analgesic effects of cryo-neurolysis, its efficacy across different patient populations has not been fully established (Biel et al. 2023). Additionally, limited data exist comparing cryo-neurolysis outcomes in bilateral versus unilateral TKA. Given the increased pain burden and prolonged recovery associated with bilateral procedures, further investigation is needed to determine whether cryo-neurolysis offers greater benefits in these patients (Manes et al. 2024). Finally, although cryo-neurolysis is generally regarded as a safe and well-tolerated procedure, long-term safety data specific to TKA patients remain scarce. Some studies report minor transient sensory deficits, but comprehensive evaluations of long-term outcomes are still lacking (Biel et al. 2023; Goyal et al. 2022).
This study serves as an initial investigation into the effectiveness of intraoperative cryo-neurolysis in TKA patients, despite the absence of a control group. We aim to evaluate its efficacy, safety, and clinical outcomes by assessing its impact on postoperative pain reduction, opioid consumption, functional recovery, and long-term pain resolution. We hypothesize that intraoperative cryo-neurolysis will lead to faster pain resolution, reduced opioid reliance, and improved early mobility compared to conventional pain management protocols. By exploring factors such as patient-specific predictors of response, its effects in bilateral versus unilateral procedures, and long-term safety considerations, this research provides valuable preliminary data to inform clinical practice and refine multimodal pain management strategies in TKA.
2. Methodology
2.1. Study Design and Patient Selection
This retrospective cohort study evaluates the impact of intraoperative cryo-neurolysis on postoperative pain management and functional recovery in patients undergoing knee arthroplasty. Data were extracted from the Tarabichi Joint Care Group single-center database for procedures performed between January 2024 and January 2025. Eligible patients were aged 50 years or older, underwent primary total knee arthroplasty (TKA) with intraoperative cryo-neurolysis, and had complete postoperative follow-up data for at least six months. Patients were excluded if they had a history of revision arthroplasty, prior knee surgery, pre-existing neurological conditions affecting pain perception, or incomplete medical records.
2.2. Surgical and Cryo-Neurolysis Procedure
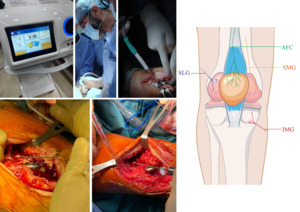
All surgeries were performed using a subvastus approach. After full implantation, wash, and tourniquet release, intraoperative cryo-neurolysis was performed using the Cryo Pain S device by Metrum Cryo Flex, a temperature-controlled cryoablation system designed for precise nerve freezing (Figure 1). The targeted nerves included the superior medial genicular nerve (SMG), inferior medial genicular nerve (IMG), superior lateral genicular nerve (SLG), and anterior femoral cutaneous nerve (AFC). Each nerve underwent two cryoablation cycles of 90 seconds each, performed at the visualized and expected anatomical locations of the targeted nerves, resulting in a total of eight cycles per case. The procedure added approximately 15 minutes to unilateral TKAs and 30 minutes to bilateral TKAs, which was accounted for in operative scheduling and workflow considerations.
2.3. Outcome Measures
The primary outcome was postoperative pain intensity, measured using the Visual Analog Scale (VAS) at multiple time points, including Day 1, Day 3, Week 1, Week 2, Month 1, Month 3, and Month 6. Secondary outcomes included functional recovery, assessed by time to first unaided ambulation and reported mobility improvements, opioid consumption, recorded as total opioid dosage in milligrams and the duration of opioid use, and postoperative complications, including infections, delayed wound healing, and nerve injury. Additionally, unplanned readmissions and reoperations were recorded over the six-month follow-up period.
2.4. Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics, Version 26.0 (IBM Corp 2017). Normality of continuous variables was tested using the Shapiro-Wilk test. Pain score trends over time were analyzed using the Friedman test, with post-hoc comparisons performed using Wilcoxon signed-rank tests. Associations between pain reduction and patient characteristics, including age, BMI, comorbidities, and operative side, were assessed using Spearman’s correlation coefficients and Mann-Whitney U tests. Differences between unilateral and bilateral TKA groups were compared using independent t-tests or Mann-Whitney U tests, depending on data distribution. A Kaplan-Meier survival analysis was performed to estimate time to complete pain resolution, defined as VAS = 0. A p-value of < 0.05 was considered statistically significant.
2.5. Ethical Considerations
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Tarabichi joint care group. Due to the retrospective nature of the study, informed consent was waived.
3. Results
3.1. Patient Characteristics
A total of 34 patients underwent knee arthroplasty with intraoperative cryo-neurolysis. The mean age was 65 ± 7 years (range: 52–78 years), with 52.9% (n = 18) male and 47.1% (n = 16) female. The majority of patients were obese (88.2%), while 11.8% were classified as overweight. Comorbidities were common, with 52.9% having diabetes mellitus, 50% with hypertension, 41.2% with hyperlipidemia, and 5.9% (n = 2) reporting a cardiac condition (Table 1).
3.2. Operative and Cryo-Neurolysis Procedure
Surgical procedures were performed unilaterally in 20 patients (58.8%) and bilaterally in 14 patients (41.2%). The mean operative time for unilateral procedures was 49 ± 4 minutes, while bilateral procedures required 93 ± 8 minutes. Cryo-neurolysis added 15 minutes for unilateral and 30 minutes for bilateral procedures (Table 2).
3.3. Postoperative Pain Scores
Postoperative pain, assessed using the Visual Analog Scale (VAS), demonstrated a statistically significant reduction over time (p < 0.00001, Friedman test, χ² = 104.8). The median pain score was 2 on Day 1 and Day 3, which decreased to 1 by Week 2 and Month 1. By Month 3, 82.4% of patients had a VAS score of 0, and by Month 6, all patients achieved complete pain relief (VAS = 0). The median time to no pain (VAS = 0) was 90 days (95% CI: 84.2–95.8 days) (Figure 2, Table 3).
All patients initially used a walker for one week, followed by a cane for two weeks before achieving unaided ambulation.
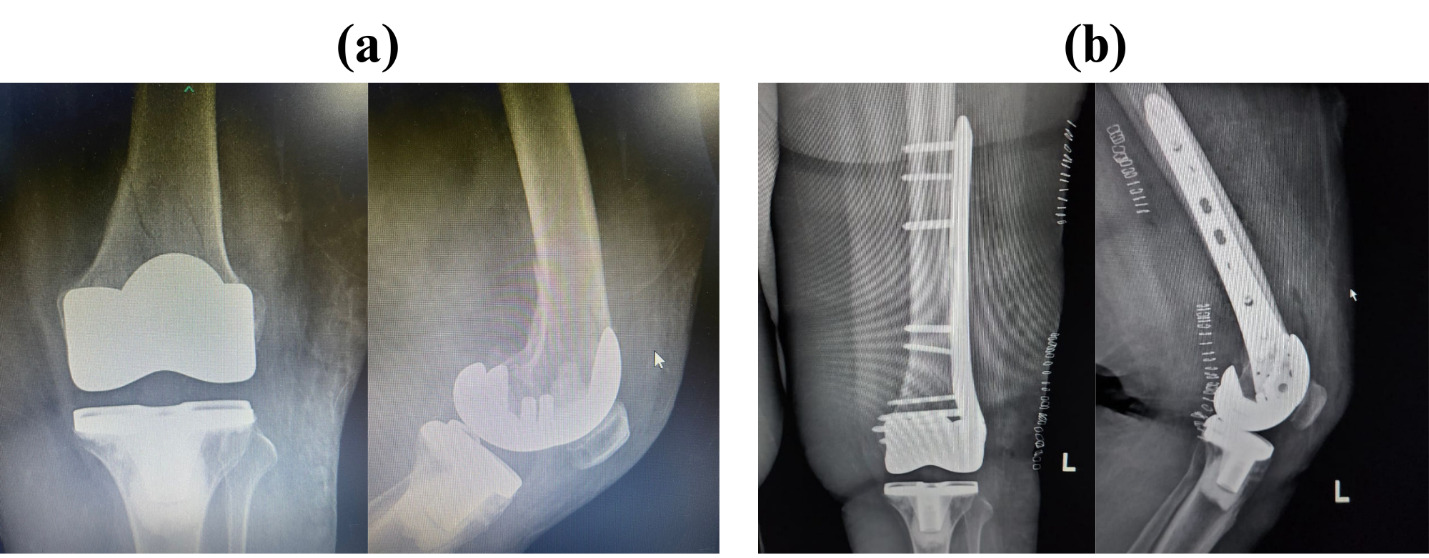
No technical complications were reported in any patient. Three patients required Tramadol 50 mg twice daily for pain management. The first patient received Tramadol on Day 1 with a pain score of 4, the second on Day 2 with a pain score of 3 on Day 1 and 2 on Day 3, and the third on Day 3 with a pain score of 2. However, one patient experienced a postoperative complication had a pain score of 4 at Week 1—a fall down the stairs two weeks after surgery, reporting a cracking sound and pain localized only to the posterior aspect of the knee. This area corresponds to the spared nerve region, suggesting that the cryo-neurolysis protocol successfully targeted the intended sensory branches without affecting deeper structures. Radiographic evaluation confirmed a fracture in the periprosthetic knee region (Figure 3).
3.4. Pain Score Trends
-
Early Postoperative Pain: On Day 1, 38.2% of patients had a VAS of 1, and 41.2% had a VAS of 2.
-
First Month Decline: By Week 2 and Month 1, 55.9% and 82.4% of patients, respectively, reported VAS = 0.
-
Sustained Pain Reduction: By Month 3 and 6, the majority had no pain, with only 17.6% reporting minimal discomfort (VAS = 1) at Month 3, which resolved by Month 6.
Over successive follow-ups, the proportion of patients reporting no change in pain scores decreased from 55.9% at Day 1 to 8.8% at Month 6, while those reporting pain reduction increased from 32.4% at Day 1 to 91.2% at Month 6. A small subset of patients experienced a transient increase in pain, peaking at Week 1 (29.4%), but resolving by Month 3 (Table 3).
3.5. Relationship Between Pain Scores and Patient Characteristics
No significant association was found between pain scores and patient demographics, comorbidities, or operative factors at any time point (Table 4).
-
Age: No correlation between age and pain reduction at any timepoint (p = 0.284 to 0.961).
-
Sex: No significant difference in pain scores between male and female patients at any time point (p = 0.657 to 0.875).
-
BMI: No significant difference in pain reduction between overweight and obese patients (p = 0.277 to 0.903).
-
Diabetes, Hypertension, Hyperlipidemia, and Cardiac Disease: These comorbidities had no significant effect on pain trajectory (p > 0.05 across all time points).
-
Operative Factors: No significant differences in pain reduction between unilateral and bilateral surgeries (p = 0.453 to 0.634), except at Month 3, where bilateral surgery showed a slightly greater pain reduction (p = 0.048).
4. Discussion
Effective postoperative pain management is a crucial component of TKA recovery. While opioid-based analgesia remains a common approach, its adverse effects—including respiratory depression, gastrointestinal complications, and the risk of long-term dependence—have led to increased interest in alternative pain management strategies (Trasolini, McKnight, and Dorr 2018; Memtsoudis et al. 2018). Cryo-neurolysis has emerged as a promising adjunct to multimodal pain management by temporarily inhibiting sensory nerve function through targeted cold application, reducing pain transmission while preserving nerve integrity (Trescot 2003). This study evaluated the efficacy of intraoperative cryo-neurolysis in improving postoperative pain control, reducing opioid use, and enhancing functional recovery over a six-month period. The findings demonstrated a statistically significant reduction in pain scores over time, with a median time to complete pain resolution (VAS = 0) of 90 days. Although comprehensive opioid consumption data were not available, the rapid decline in pain scores suggests a reduced reliance on analgesics. Additionally, no significant associations were found between pain reduction and patient-specific factors, indicating that cryo-neurolysis is effective across diverse patient populations.
Research on cryo-neurolysis in TKA has primarily focused on preoperative cryo-neurolysis, typically applied 3–7 days before surgery to the superficial genicular nerves. While these studies have reported reductions in opioid consumption and improved early pain control, their long-term efficacy remains debated. A clinical trial investigating preoperative cryo-neurolysis did not demonstrate a statistically significant reduction in cumulative opioid consumption over a six-week follow-up period (William M. Mihalko et al. 2021). Similarly, Wickline, Terentieva, and Cole (2024) found no significant reduction in opioid use in TKA patients who underwent preoperative cryo-neurolysis, further highlighting the need for alternative application methods.
In contrast, our study assessed intraoperative cryo-neurolysis, which offers potential advantages, including direct visualization and precise nerve targeting. While the literature on intraoperative cryo-neurolysis remains limited, studies on cryoanalgesia in chronic pain management have demonstrated sustained analgesic effects lasting up to 12 weeks (Dasa et al. 2016; W. M. Mihalko et al. 2019). This aligns with our findings, where pain scores showed a steady decline, reaching a median VAS of 0 by Month 3, with 82.4% of patients reporting complete pain resolution at that point. This suggests that intraoperative cryo-neurolysis may provide a more consistent and prolonged analgesic effect compared to preoperative application.
Intraoperative cryo-neurolysis offers several advantages over closed cryoablation techniques. First, it requires significantly less time (approximately 15 minutes) than preoperative closed cryoablation. Second, reusable probes can be utilized without the risk of damaging the shanks or experiencing blockage due to bending, a common limitation in the closed approach. Third, intraoperative application allows for direct visualization and full contact of the probe along the targeted genicular branches, ensuring consistent nerve treatment. Finally, soft tissue structures can be effectively retracted during the procedure, reducing the risk of unintended injury to surrounding tissues.
Other non-opioid pain management modalities, such as continuous cryotherapy and peripheral nerve blocks, have been explored in TKA. Continuous cryotherapy has shown benefits in reducing both postoperative pain and opioid consumption (Wyatt et al. 2023). However, its effectiveness is largely limited to the immediate postoperative period, whereas cryo-neurolysis provides prolonged pain relief lasting several weeks to months (Nygaard et al. 2021). Peripheral nerve blocks, including adductor canal and femoral nerve blocks, remain widely used due to their established efficacy in postoperative pain control. However, these techniques carry risks of motor blockade, which can delay early mobilization and rehabilitation (Ilfeld, Gabriel, and Trescot 2017). In contrast, cryo-neurolysis selectively targets sensory nerves, preserving motor function while offering sustained analgesia without the need for continuous catheter-based drug administration.
Postoperative cryotherapy is a common modality employed to mitigate pain and swelling following TKA. A systematic review and meta-analysis encompassing 11 randomized controlled trials with 793 TKAs assessed the efficacy of cryotherapy in this context. The findings indicated that while cryotherapy led to minor benefits in blood loss and discharge knee range of motion, it did not significantly impact transfusion and analgesia requirements, pain levels, swelling, length of hospital stay, or long-term gains in knee range of motion post-discharge. The authors concluded that despite some early advantages, cryotherapy after TKA does not yield substantial lasting benefits, and patient-centered outcomes remain underexplored(Adie, Naylor, and Harris 2010).
In contrast, recent studies on cryoneurolysis have demonstrated promising results(Manes et al. 2024; Mont et al. 2025; Lung et al. 2022). A retrospective chart review comparing TKA patients who received preoperative cryoneurolysis to age-matched controls found that the cryoneurolysis group exhibited reduced inpatient and outpatient opioid consumption, improved knee range of motion at six weeks, and enhanced patient-reported outcomes at one-year follow-up. Importantly, there were no significant differences in complication rates between the groups(Lung et al. 2022).
This study included 34 patients, with a high prevalence of obesity (88.2%) and comorbidities such as diabetes (52.9%) and hypertension (50%). Despite these factors, pain reduction patterns remained consistent across all subgroups, suggesting that cryo-neurolysis is broadly effective and does not require strict patient selection criteria. This is particularly important given the growing number of TKA patients with obesity and metabolic disorders, who often experience more severe postoperative pain and delayed recovery (Biel et al. 2023). The additional operative time required for cryo-neurolysis was minimal (15 minutes for unilateral and 30 minutes for bilateral TKA), making it a feasible addition to surgical workflows.
Pain scores demonstrated a statistically significant decline (p < 0.00001) over the study period. On Day 1 and Day 3, median pain scores were 2, decreasing to 1 by Week 2 and Month 1, and reaching 0 by Month 3. The proportion of pain-free patients increased from 5.9% at Week 1 to 82.4% at Month 3, confirming the sustained benefit of cryo-neurolysis. The estimated median time to complete pain resolution was 90 days (95% CI: 84.2–95.8 days). These results provide strong evidence that cryo-neurolysis accelerates postoperative pain resolution, particularly in the critical early recovery period.
Interestingly, no significant correlation was observed between pain scores and age, sex, BMI, diabetes, hypertension, hyperlipidemia, or cardiac disease at any postoperative time point. This finding suggests that cryo-neurolysis provides uniform pain relief across a heterogeneous patient population. One notable finding was that patients undergoing bilateral TKA exhibited greater pain reduction at Month 3 compared to unilateral TKA patients (p = 0.048). Given the higher pain burden and longer recovery associated with bilateral procedures, this suggests that cryo-neurolysis may provide even greater benefits in patients undergoing more extensive surgical interventions (Manes et al. 2024).
The implementation of intraoperative cryoneurolysis introduces additional time in the operating room (OR), which has economic implications (Lung et al. 2022). Given that OR time is a significant cost driver in surgical procedures, the added duration required for cryoneurolysis must be justified by its clinical benefits. Studies have reported that patients undergoing cryoneurolysis experience reduced opioid consumption and improved functional outcomes, which can potentially lead to shorter hospital stays and decreased overall healthcare costs (Lung et al. 2022). In our study, the observed reduction in postoperative pain scores suggests that the anticipated decrease in opioid use may help justify the additional OR time. To further enhance the cost-effectiveness of this technique, efforts are underway to reduce the additional OR time by utilizing modified probes and streamlining the procedure. Future research should focus on conducting comprehensive cost-benefit analyses to fully assess the economic impact of integrating cryoneurolysis into standard TKA protocols.
These findings suggest that cryo-neurolysis should be considered as a standard adjunct in multimodal pain management protocols for TKA. By providing sustained postoperative pain relief, it has the potential to reduce opioid dependence, minimize side effects, and facilitate earlier rehabilitation. The lack of association between cryo-neurolysis efficacy and patient comorbidities suggests that it can be widely implemented without strict patient selection criteria, making it a versatile option for pain management in TKA patients. Furthermore, the minimal additional surgical time required (15–30 minutes) supports its feasibility for integration into routine TKA procedures.
This study is limited by its retrospective design, which introduces potential selection bias. Additionally, the absence of a control group prevents direct comparisons, making it difficult to isolate the specific analgesic effects of cryo-neurolysis from the natural postoperative pain trajectory. Opioid consumption data were inconsistently recorded, further limiting the ability to assess its impact on analgesic use. The study was also conducted at a single center with a relatively small sample size (n = 34), which restricts generalizability. However, as this technique is novel, larger, prospective, randomized controlled trials are warranted to validate these findings, explore long-term safety and efficacy, and optimize its clinical application in TKA patients.
5. Conclusion
This study highlights the potential of intraoperative cryo-neurolysis as a highly effective adjunct in multimodal pain management for TKA patients. The findings demonstrate significant, sustained pain reduction, with the majority of patients reporting no pain by three months postoperatively. Importantly, cryo-neurolysis was effective across a diverse patient population, regardless of comorbidities, and did not require strict patient selection criteria. The minimal additional surgical time required (15–30 minutes) supports its integration into routine TKA procedures without significant operational disruptions.
While the study provides strong evidence for the clinical benefits of cryo-neurolysis, including opioid reduction, accelerated pain resolution, and improved functional outcomes, further research is needed. Larger, prospective, randomized controlled trials are essential to validate these findings, assess long-term safety, and establish standardized protocols for its use in clinical practice.