Introduction
The presence of bone bioburden following sterile processing presents a scenario where there is uncertainty regarding sterility of adjacent instruments and trays. Theoretically, all instruments processed within the autoclave itself are at risk due to the circulation of steam during the cycle. The use of a multi tray pod allowed a controlled method of assessing adjacent instrument contamination in the presence of post processing bone bioburden.
Prior studies have investigated the sterility of bioburden that has been processed using an autoclave only process (Resendiz et al. 2020; Smith et al. 2018; Mayer et al. 2016). This is the first study to the authors’ knowledge that investigates bone bioburden in the context of the standard steps used at most medical centers for the processing of orthopedic instruments. The goal was to replicate a real world scenario where each step of the sterile processing has been performed in accordance with standard guidelines (ANSI/AAMI ST79:2017/(R)2022, n.d.). We chose G. stearothermophilus as the contaminating organism due to its thermophilic nature and spore forming properties. This represents a worst case organism and is the species generally chosen for validation and certification of sterile processing systems (ANSI/AAMI ST79:2017/(R)2022, n.d.; ANSI/AAMI/ISO 14937:2009/(R)2013, n.d.; AAMI TIR39:2009/(R)2017, n.d.). The addition of multi-tray processing during this testing allowed for a thorough assessment of adjacent instrument and tray contamination. These are in use currently throughout various medical centers in the United States and the authors institution since 2016. The appeal of the multi tray processing pod is the ability to reduce waste, reduce processing man hours, and reduce turn over time (Bradley et al. 2021). One concern regarding multi-tray processing is the limited experimental data regarding bioburden and adjacent instrument contamination risk. This study was structured by practicing academic orthopedic surgeons and the testing performed at a private laboratory specializing in sterile processing certification.
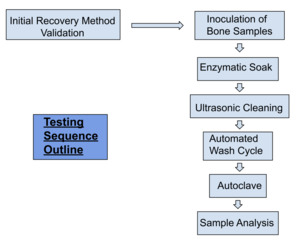
Methodology
An initial validation was performed using porcine bone and commercially available G. stearothermophilus. A mixture of cortical and cancellous porcine bone was inoculated and 1x10^6 Colony forming units were verified to be recovered using the below listed culture and indicator testing methods (ANSI/AAMI/ISO 11138-7:2019, n.d.). The approximate size of the bone was 4-6 mm in size. The initial validation was to ensure that an appropriate recovery method was selected for the porous and irregular surface of the bone.
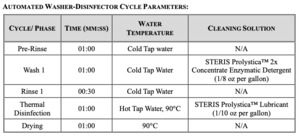
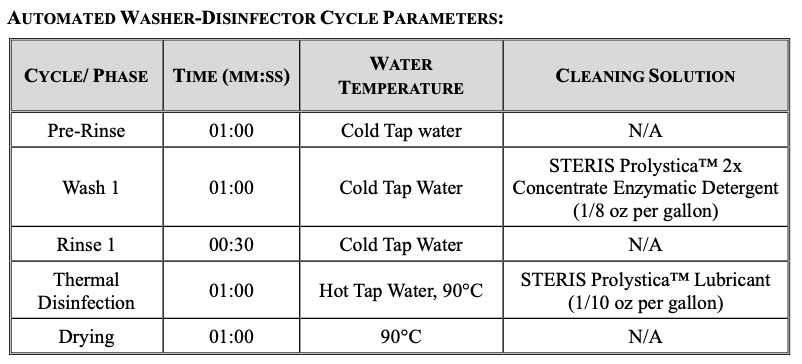
Three cycles of testing were performed in total. Each cycle consisted of twelve porcine bone test samples that were placed into wire mesh stainless-steel tea strainers, one bone sample per strainer. Each sample was inoculated with the commercially available G. stearothermophilus. The stainless steel strainers were used to ensure the bioburden remained present throughout the testing process. The inoculated bone remained within the strainers for the entirety of the test procedure until transferred to culture media. The instrument trays were processed containing the bone test samples in a standard manner which followed the healthcare sterile processing standards at our academic medical center. The trays and bone test samples were immersed into Prolystica™ Enzymatic cleaning solution and they were allowed to soak for five minutes at 30-35°C. The samples were then placed into an ultrasonic cleaner with the same preparation of Prolystica™ solution and sonicated for ten minutes. The bone samples were rinsed under warm running tap water for one minute to remove the residual detergent and dried with compressed air. The cycle tapes were inspected to verify that the parameters were met per the washer disinfector cycle listed.
TEST TRAY LOADING CONFIGURATION
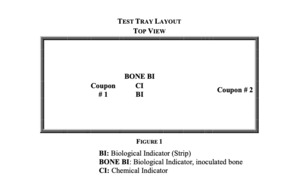
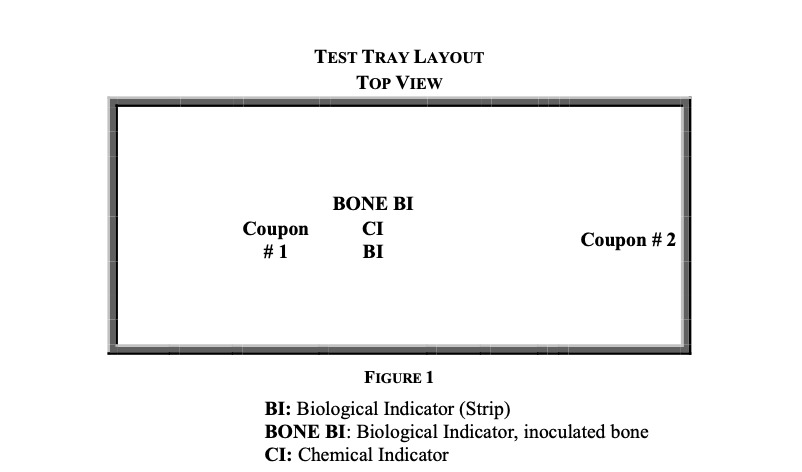
Twelve perforated sterilization trays were obtained and loaded with medical and simulated medical devices to have a combined minimum tray and instrument weight of twenty-five pounds. The washed and dried bone test samples remained in the strainers and were placed in the center of the test trays with a chemical indicator. Two stainless-steel coupons were placed in each tray. The coupons are rectangular stainless steel plates used to simulate a medical instrument with a large surface area and are commonly used in certification procedures for sterile processing (ANSI/AAMI/ISO 14937:2009/(R)2013, n.d.). One coupon was placed adjacent to the inoculated bone test samples and the other coupon located at the edge of the tray, Fig. 1.
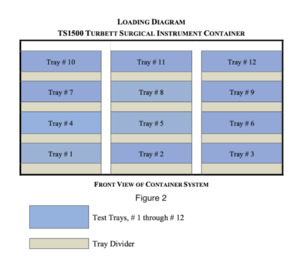
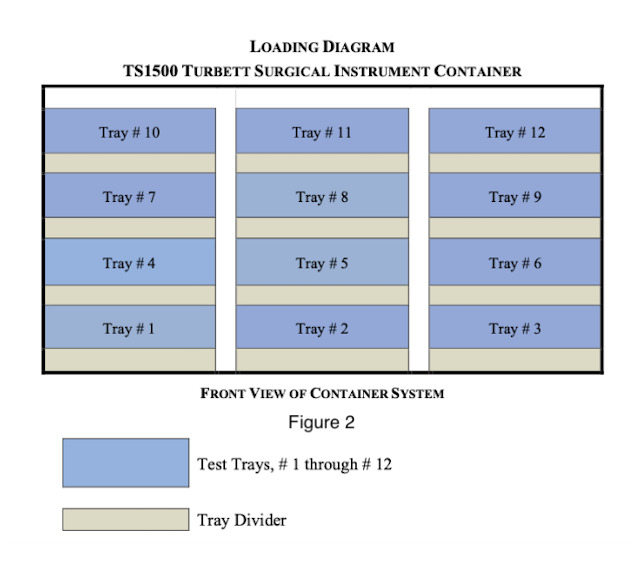
The multi-tray sterilization pod was manufactured by Turbett Surgical. The exact model used for testing was the TS1500 Instrument Pod and is approximately 34x22x24 inches in size. Each processing pod is designed to hold twelve trays without wrapping. Tray dividers were positioned within the base of the Instrument Pod and between each level of trays until all twelve trays were loaded into the instrument pod. The trays were unwrapped and an outer screen to serve as a filter was used as per the manufacturer recommendations. See the below listed diagram for orientation, Fig. 2.
The pod was pushed into the sterilizer until it was over the drain. The drain has been identified as the coldest location within the sterilizer chamber as determined by the sterilizer manufacturer. The pod was processed using the steam pre-vacuum sterilization cycle listed below and in accordance with ANSI/AAMI/ISO 14937:2009/(R)2013 and ANSI/AAMI ST79:2017/(R)2022. Following cycle completion, the pod was removed from the sterilizer and the pod was moved into the laminar flow hood room.The sterilizer cycle tape was reviewed to verify the cycle parameters were achieved.
TRANSFER PROCEDURE
The proper sterility testing attire was donned, including sterile gloves, hair covers, surgical masks, and gowns before proceeding. The entire transfer procedure was performed utilizing aseptic technique. An environmental control was established by uncapping and exposing one vessel of culture media within the laminar flow hood for the duration of the transfer period. The test trays were removed into the pre-cleaned laminar flow hood. The bone test samples and coupons were transferred to separate, individually labeled, vessels of culture media. The chemical integrator was observed for steam penetration. A positive control was established by inoculating media from the batch used in testing with an un-cleaned and un-sterilized bone test sample. An additional positive control was established for the lot of spore strips used in testing. The bone test samples, coupons, and controls were incubated at 55-60°C for a minimum of seven days. A total of 3 test cycles were performed and the test results listed in the table below, Table 1. There were no positive test samples and therefore no Gram-stain was required. The above testing was done in accordance with ANSI/AAMI/ISO 11138-7:2019.
RESULTS
The biological indicator, inoculated bone, spore strips, and coupons were negative for growth of the target organism (G. stearothermophilus) following the minimum incubation period of seven days ANSI/AAMI/ISO 11138-7:2019. The positive controls were positive for growth of the target organism (G. stearothermophilus) following the minimum incubation period of seven days. The negative and environmental controls were negative for growth of the target organism (G. stearothermophilus) following the minimum incubation period of seven days. The chemical integrator demonstrated steam penetration with an acceptable color change of white to blue into the “safe” zone of the indicator. The washer-disinfector and steam sterilizer cycle tapes were verified and showed that the appropriate parameters listed above were achieved.
TESTING RESULTS
Discussion
Sterile processing of instruments continues to be a complex environment due to the nature of instruments processed (Lopes et al. 2019), and variations in manufacturer recommendations as required by ANSI/AAMI/ISO 17664-1:2022 and AAMI TIR12:2020. These instrument specific requirements rely heavily on the individual departments’ interpretation of the various manufacturer recommendations (Brooks, Williams, and Gorbenko 2019). This results in considerable variation in methods for meeting the standards required by ANSI/AAMI/ISO.
Prior reports of focused sterile processing department standardization and validation have shown positive results in reducing surgical delays and improving overall efficiency (Huynh et al. 2019; Cichos et al. 2019). Ideally, automation of the validation process would reduce error further and provide objective evidence of sterilization steps. This then brings the possibility of implementing policies that reflect the actual risk of bioburden presence after full processing through the use of a validation protocol. A reduction in reprocessing, surgical delay, and increased surgical times would have dramatic monetary effects and potentially reduced postoperative complications. Concerns about bioburden after sterile processing are warranted given the dramatic effect that hospital acquired infections have on patients and the healthcare system (Marchetti and Rossiter 2013). One study performed a random inspection of depth gauges after sterile processing at their trauma center. Alarmingly, greater than ninety percent of the instruments inspected contained visible bioburden (Wanke et al. 2018). Delays in surgical procedures, increased surgical times, and reprocessing are a burdensome part of contemporary practice. In a study that looked at surgical procedure stoppage immediately before incision they found that over fifty percent were a result of supply and instrument management (Coudane et al. 2018). Bone bioburden presence after sterile processing represents an area for systems improvement and requires further analysis to build policies that improve safe and effective surgical care. It should also be emphasized that regardless of the results of this study, the removal of bone bioburden from instruments during processing should still be undertaken as routine practice. Our study suggests that reprocessing of entire autoclave cycles or multi tray pods is likely to not be necessary in the presence of visible bone.
The study was conducted at a research facility that performs certification and validation testing. Reproduction of this study design in various settings would be helpful to generalize the overall risk profile of bone bioburden. All efforts were made to create as much of a real world scenario in the highly controlled laboratory setting. Additionally, the highly controlled laboratory setting poses a potential limitation when compared to real world sterile processing departments.
Conclusions
This is the first investigation to the authors’ knowledge that examines bone bioburden in the context of a standard full sterile processing sequence. Similar prior studies have focused on the autoclave only efficacy. This study suggests that bone bioburden that has been through the full sequence of sterile processing is not contaminated and does not cross contaminate adjacent instruments or trays. The methodology used most closely replicated the scenario of freely exposed bone bioburden. Future studies to evaluate the effectiveness of a standard sterilization process on bioburden entrapped in cannulated instruments are necessary.