Introduction
Proximal hamstring tendon injuries are commonly seen in athletes from a variety of sports and are often the result of rapid hip flexion while the knee is extended (Lightsey et al. 2018; Miller, Gill, and Webb 2007). These injuries are typically classified using a 3-level grading scale based on magnetic resonance imaging (MRI) findings, with higher grades indicating a more severe injury. Grade 1 injuries involve <5% of the muscle and are consistent with a muscle strain. Grade 2 injuries, which are partial tears, involve 5%–50% of the tendon thickness. Grade 3 injuries are complete tears or avulsions involving 100% tendon thickness (Heer et al. 2019). Injuries can vary in terms of which hamstring muscles are involved or the specific injury site (either muscle-tendon junction or tendon-bone junction). The treatment of proximal hamstring tendon injuries depends on the severity. Nonoperative treatment is indicated if no tear is present or if the tear involves only a single tendon with minimal retraction. This treatment typically consists of rest, ice, nonsteroidal anti-inflammatory drugs (NSAIDs), and limited weight-bearing for up to 4 weeks (Guanche 2015). Operative treatment is indicated if there is a two-tendon tear with >2 cm of retraction or if the tear/avulsion involves more than two tendons (Miller, Gill, and Webb 2007; Guanche 2015).
Hamstring repair involves reattaching the avulsed tendons to the ischial tuberosity with suture anchors and this can be performed either open or endoscopically (Miller, Gill, and Webb 2007). Operative treatment is further classified into acute and chronic repairs. Acute repair is usually defined as surgical treatment of the avulsed tendon(s) performed within 4 weeks of injury, while chronic repair occurs more than 4 weeks after the injury (Cohen et al. 2012; Harris et al. 2011; Moatshe, Chahla, Vap, et al. 2017).
Several complications can develop following operative treatment of proximal hamstring injuries including re-rupture, reoperation, surgical wound dehiscence, sciatica-type pain, weakness, repair failure, wound infection, and deep vein thrombosis (DVT) (Bodendorfer, Curley, Kotler, et al. 2018; Bowman et al. 2019; Van der Made et al. 2015). The most common complications include peri-incisional numbness (5.4%) and neurologic problems (8%), while re-rupture, reoperation, and infection/wound complications occur at rates of 2.2%, 2.6%, and 3.3%, respectively (Bodendorfer, Curley, Kotler, et al. 2018). Scarring of the sciatic nerve can occur following proximal hamstring repair if the nerve bundle is not well-protected. Chronic hamstring tears can also lead to scarring potentially from the injury itself (Bodendorfer, Curley, Kotler, et al. 2018). DVT remains a concern following repair of the proximal hamstring tendons, yet the rate of occurrence of this potential post-operative complication has not been previously characterized. The purpose of this study was to characterize the factors related to the development and treatment of DVTs following proximal hamstring repair. We hypothesized that DVT would occur more commonly following repair of chronic proximal hamstring tendon avulsion injuries compared to acute injuries.
Materials and Methods
A systematic review of the literature was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al. 2021). A search of the PubMed and EMBASE databases was performed by two independent reviewers from database origin until May 2022. The following search terms were used: “tear”, “avulsion”, “rupture”, and “proximal”. MeSH and title/abstract (tiab) terms were also utilized in the search: “hamstring muscles [MeSH]”, “hamstring tendons [MeSH]”, “hamstring[tiab]”, “semimembranosus[tiab]”, “semitendinosus[tiab]”, “biceps femoris[tiab]”, “femoral biceps[tiab]”. Boolean operators “OR” and “AND” were used to combine synonyms and categories. A subsequent database search was performed in May 2023, which did not populate any further articles to be included in this review.
Titles and abstracts were screened to assess eligibility based on inclusion/exclusion criteria. Studies were included if they discussed surgical repair of proximal hamstring injuries, contained details on postoperative outcomes for a period of at least 6 months, contained a detailed description of the acute or chronic nature of the injury, included a description of the number of tendons injured, and patient demographics (e.g., age, gender, time since injury, mechanism of injury). Studies were excluded if they were case reports, review articles, anatomic reviews, surgical technique reviews, cadaveric studies, were not published in English, and focused on muscle groups other than the hamstrings. Studies that did not discuss surgical complications were also excluded. The full texts of included studies were reviewed by two authors, and any discrepancies were resolved by a third author. The following information was extracted: patient demographics, type of hamstring tendon injury, complications following repair, DVT prophylaxis, and DVT treatment. Risk of bias was assessed according to the ROBINS-I risk of bias tool for the included non-randomized studies, which incorporates an assessment of bias due to confounding, selection of participants, deviations from intended interventions, completeness of outcomes data, selection of outcomes reported, and other sources of bias.
Results
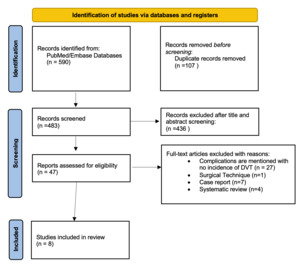
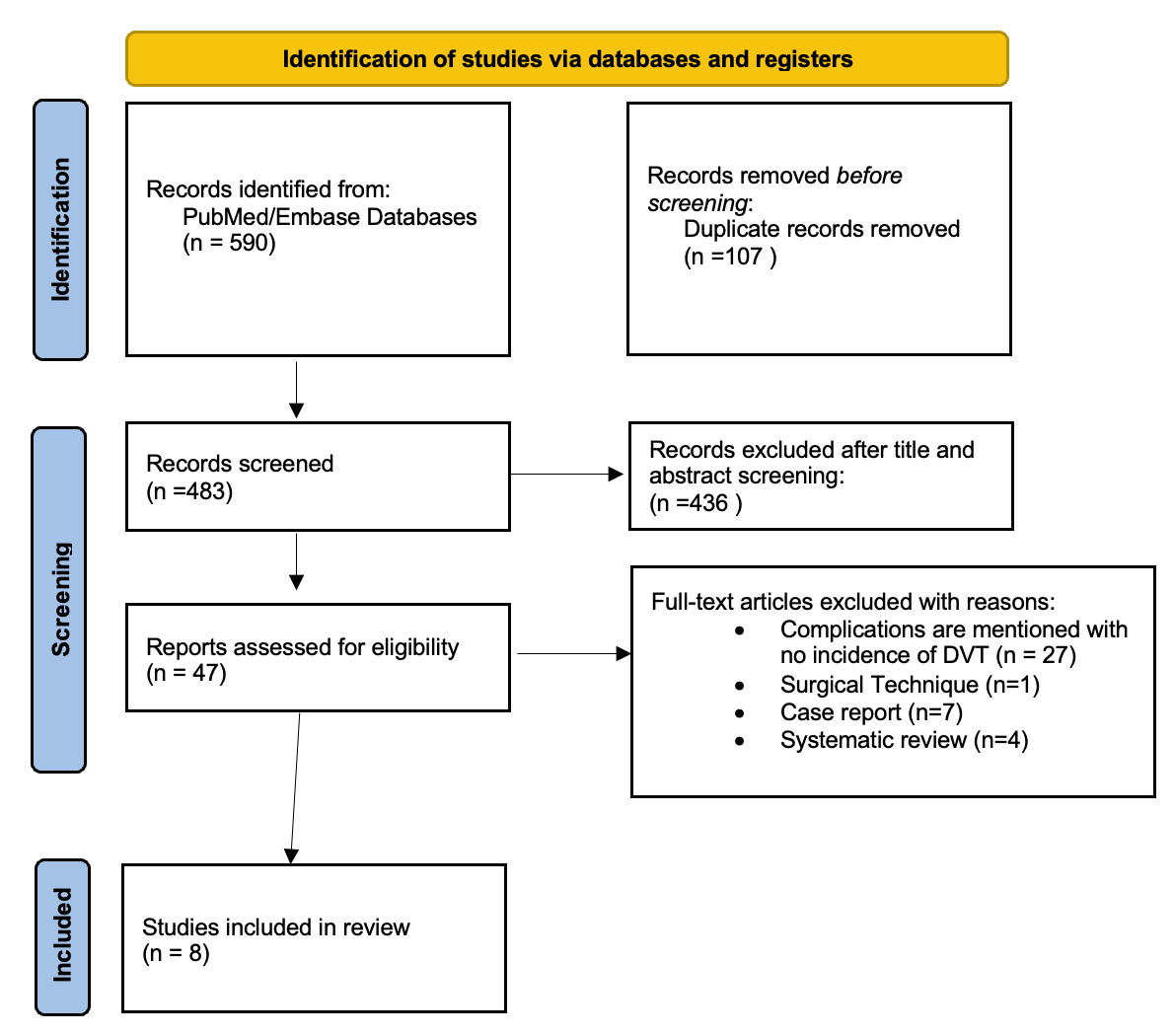
The initial search yielded a total of 590 studies. After removing duplicates, 483 studies remained. Titles and abstracts were screened, and 436 studies were removed for not meeting the inclusion criteria, leaving 47 studies for full text review. Of those 47 studies, 8 specifically commented on DVT as a complication of proximal hamstring repair and were included (Figure 1). From these 8 non-randomized studies, 7 (87.5%) were case series (level IV evidence), and 1 (12.5%) was a therapeutic trial (level III evidence) (Table 1).
While surgical technique varied for all 8 studies, they each utilized an open repair. Only one study (12.5%) documented the use of DVT prophylaxis (Cohen et al. 2012). All 52 patients (26 male; 26 female) undergoing proximal hamstring repair were prescribed four weeks of aspirin postoperatively. No note was made of dose or compliance for any patient, including the patient who developed a DVT (Cohen et al. 2012). From the 8 studies included in this review, a total of 9 cases of DVT were identified in the postoperative period, which made up 2.1% (9/422) of the study population or 1.9% (9/464) of the surgical cases. All 9 (100%) of the DVTs occurred following an open repair. Additionally, all patients who were found to have a DVT were subsequently treated with anticoagulation therapy, with successful resolution of the DVT.
DVT Incidence
Willinger et al. reported 2 cases of DVT in their study in patients who had an acute, complete tear of their proximal hamstring tendon (Willinger et al. 2019). Whereas, in a 2008 study by Sallay et al., a patient who had undergone repair for chronic proximal hamstring tendon rupture was diagnosed two weeks postoperatively with a DVT (Sallay et al. 2008). According to their study, the patient lived out of town and after flying home he became symptomatic. He was subsequently treated with anticoagulant therapy by their home physician and recovered fully. The study did not specify which anticoagulant was used or length of treatment time. Ebert et al. examined outcomes in patients following repair of a chronic proximal hamstring tendon avulsion and reported one occurrence of DVT earlier in the postoperative period and recovered with no further complications (Ebert, Gormack, and Annear 2019). A study conducted by Sarimo et al. reported one case of DVT that was successfully treated with anticoagulant therapy, such that symptoms had subsided by final follow up (Sarimo et al. 2008). There were no specifics given regarding which type of anticoagulant was used nor how long after surgery the DVT occurred. One patient in the study by Cohen et al. developed a DVT at an unspecified time following surgery and was treated with unspecified anticoagulant therapy for 3 months (Cohen et al. 2012). The patient had received four weeks of aspirin for DVT prophylaxis. Léger-St-Jean et al. reported one instance of DVT in their study that occurred in the contralateral leg but did not specify treatment or length of time between surgery and diagnosis (Léger-St-Jean et al. 2019). A patient included in the study performed by Kanakamedala et al. developed a DVT 6 weeks postoperatively and was successfully treated with unspecified anticoagulation therapy of unknown duration (Kanakamedala et al. 2022). They stated that this patient had a history of a superficial deep venous thrombosis. In the study by Willinger et al., two patients developed DVTs following repair of an acute tendon tear (Willinger et al. 2019). The acute/chronic nature of the tear was not defined for the other 5 patients (55.6%) who experienced a DVT. In 2014, Barnett et al. reported a DVT in one patient following a proximal hamstring repair. This was successfully treated with warfarin for an unknown period of time (Barnett, Negus, Barton, et al. 2015). In 2 (25%) of the studies, a patient was diagnosed with a DVT and a pulmonary embolism (PE) (Barnett, Negus, Barton, et al. 2015; Kanakamedala et al. 2022). In one study, the PE and DVT were treated with warfarin for an unspecified duration, which resulted in resolution. It was not specified whether the duration of warfarin treatment was altered by the presence of the DVT with concomitant PE. The patient subsequently developed a hematoma requiring surgical intervention (Barnett, Negus, Barton, et al. 2015). The other study did not specify anticoagulant used or duration of use (Kanakamedala et al. 2022).
Surgical Technique
Surgical technique was described in each of the 8 studies. Cohen et al. and Léger-St-Jean et al. reported the use of a transverse incision (Cohen et al. 2012; Léger-St-Jean et al. 2019). Barnett et al., Kanakamedala et al., Sarimo et al., Willinger et al., Sallay et al., and Ebert et al., utilized longitudinal incisions (Barnett, Negus, Barton, et al. 2015; Kanakamedala et al. 2022; Sarimo et al. 2008; Willinger et al. 2019; Sallay et al. 2008; Ebert, Gormack, and Annear 2019). Studies varied in the number of suture anchors used. For example, the procedure included in studies conducted by Kanakamedala et al., Léger-St-Jean et al., Sarimo et al., Willinger et al, and Sallay et al. used two anchors, while Cohen et al. stated that their surgical technique included the use of 5 anchors (Kanakamedala et al. 2022; Léger-St-Jean et al. 2019; Sarimo et al. 2008; Willinger et al. 2019; Sallay et al. 2008; Cohen et al. 2012). Cohen et al., Barnett et al., Kanakamedala et al., Léger-St-Jean et al., Sarimo et al., Willinger et al., and Sallay et al. used suture anchors to reattach the torn or avulsed tendon directly to the ischial tuberosity (Cohen et al. 2012; Barnett, Negus, Barton, et al. 2015; Kanakamedala et al. 2022; Léger-St-Jean et al. 2019; Sarimo et al. 2008; Willinger et al. 2019; Sallay et al. 2008).
Patient Outcomes
One study (12.5%) examined the use of gracilis and semitendinosus tendons harvested from the ipsilateral distal hamstrings to attach the proximal hamstring stump to the suture anchors in the ischial tuberosity (Ebert, Gormack, and Annear 2019). All 6 patients were undergoing proximal hamstring repair for a chronic, retracted tear. Five of 6 (83.3%) patients were satisfied at 24 months postoperatively. The other 7 studies (87.5%) that used a suture anchor to reattach the torn or avulsed tendon directly to the ischial tuberosity included a mix of patients with chronic (168/420, 40%) and acute (246/420, 58.6%) injuries. Cohen et al. reported that 51 of 52 (98.1%) patients were satisfied with postoperative outcomes regarding function after proximal hamstring repair based on subjective questionnaire (Cohen et al. 2012). Similarly, Sallay et al. conducted a study evaluating patient-based results after a proximal hamstring repair via subjective questionnaire that showed 100% (25/25) of their patients were content with their progress following the surgery (Sallay et al. 2008). Léger-St-Jean et al. did not report satisfaction rates but did report Lower Extremity Functional Scale (LEFS) and Single Assessment Numeric Evaluation (SANE) scores of 85.7 + 21.3 and 86.9 + 11.7, respectively (Léger-St-Jean et al. 2019). Two studies did not report patient satisfaction rates, but Willinger et al. measured postoperative outcomes via Lysholm score, Harris Hip Score (HHS), Tegner activity scale, Visual Analog Scale (VAS), and Return to sport (RTS) (Willinger et al. 2019). Kanakamedala et al. also did not report satisfaction rates, but included Perth Hamstring Assessment Tool (PHAT), Modified Harris Hip Score (MHHS), Visual Analogue Scale for pain (VAS), Pre-injury Tegner, and Post-operative Tegner scores (Kanakamedala et al. 2022).
Risk of Bias Assessment
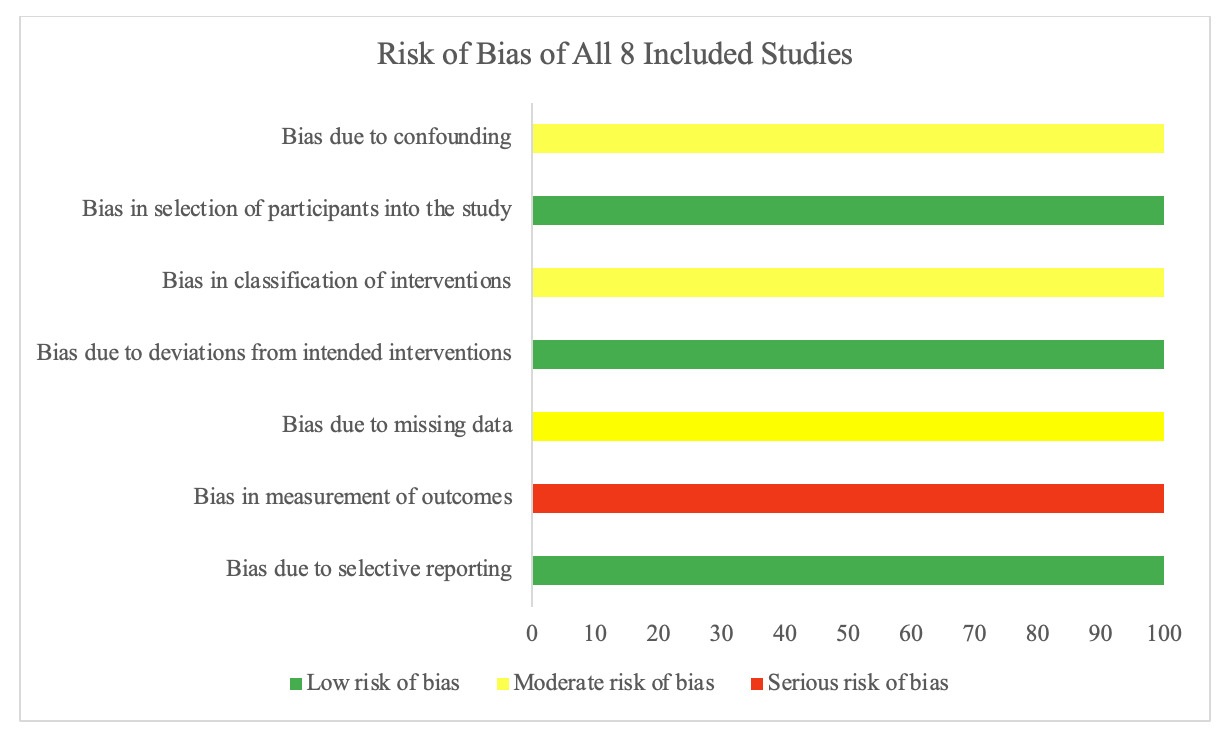
The results of the risk of bias assessment of the 8 included studies using the ROBINS-I risk of bias tool are presented in Figure 2 (Sterne, Hernán, Reeves, et al. 2016). All 8 studies presented a moderate risk of bias due to confounding, as there were no prognostic variables used to predict baseline intervention. No patients switched between interventions during the study period, as the only other alternative would have been nonoperative treatment (low risk of bias). No eligible patients were excluded from any of the studies, thus presenting low risk of bias due to selection of participants. None of the studies deviated from the intended intervention, and no studies used variable follow-up times (low risk of bias) (Sterne, Hernán, Reeves, et al. 2016). All 8 studies clearly classified treatment type; however, post-operative protocols for DVT prophylaxis were not specified in 7 studies (Barnett, Negus, Barton, et al. 2015; Kanakamedala et al. 2022; Léger-St-Jean et al. 2019; Sarimo et al. 2008; Willinger et al. 2019; Sallay et al. 2008; Ebert, Gormack, and Annear 2019). However, one study mentioned that they utilized 4 weeks of post-operative aspirin at an unspecified dose (moderate risk of bias) (Cohen et al. 2012). All eight studies used physicians not blinded to the treatment group (serious risk of bias [Cohen et al. 2012; Barnett, Negus, Barton, et al. 2015; Kanakamedala et al. 2022; Léger-St-Jean et al. 2019; Sarimo et al. 2008; Willinger et al. 2019; Sallay et al. 2008; Ebert, Gormack, and Annear 2019]. Finally, no studies showed bias due to selective reporting (low risk of bias).
This review used MINORS criteria shown in Table 2 to examine the quality of non-randomized studies included in this paper (Slim et al. 2003). The scoring system had 12 individual elements; however, only comparative studies were evaluated in items 9-12. All eight studies used in this review were scored 0 if not reported, 1 if reported but not adequate, and 2 if reported and adequate. The maximum possible score for non-comparative studies was 16 and 24 for comparative studies. Based on the results of Table 2, Barnett et al., Ebert et al., and Sarimo et al. received a score of 16; Cohen et al. received a score of 15; Sallay et al., received a score of 13; and Kanakamedala et al., Léger-St-Jean et al., and Willinger et al. received a score of 23 (Cohen et al. 2012; Barnett, Negus, Barton, et al. 2015; Kanakamedala et al. 2022; Léger-St-Jean et al. 2019; Sarimo et al. 2008; Willinger et al. 2019; Sallay et al. 2008; Ebert, Gormack, and Annear 2019).
Discussion
While previous studies have characterized the complications associated with repair of proximal hamstring injuries, this is the first systematic review to focus specifically on the incidence of DVT in this patient population. We identified 47 studies that discussed complications following repair of the proximal hamstring, 8 of which specifically evaluated the rate of DVT. All 8 of these studies had at least one patient that developed a DVT following proximal hamstring repair. The most DVTs occurred in patients with a complete proximal hamstring tendon avulsion, though the incidence was low. Based on the studies reviewed in this paper, it can be determined that the risk of post-operative DVTs is low compared to other post-operative complications evaluated.
In 2018, Bodendorfer et al. performed a meta-analysis for treatment of proximal hamstring avulsions and associated complications (Bodendorfer, Curley, Kotler, et al. 2018). The authors found an overall incidence of DVT or PE in 5 of the 767 patients (0.68%) operatively treated patients that were assessed. No distinction was made between DVT or PE in this regard. Four of the 5 (80%) patients who developed a DVT/PE had no information on the nature of the injury, while one patient’s DVT occurred following repair of a complete, chronic avulsion. Two other DVT/PE patients (40%) had complete proximal hamstring avulsions; therefore, a total of 3 out of 5 identified DVT/PE cases (60%) occurred in the setting of a complete avulsion. The complete vs partial nature of the remaining 2 DVT/PE patients (40%) was not known (Bodendorfer, Curley, Kotler, et al. 2018). Similarly, our study found no clear association between the complete vs partial nature of the injury and the occurrence of a DVT. We also identified a similar chronicity of hamstring injury among the DVT patients, which may be the result of a prolonged surgical time or increased effort to mobilize the tendon with respect to an acute tendon injury.
A systematic review performed by van der Made in 2015 reported that of the 387 patients who underwent surgical treatment of proximal hamstring injuries, 3 developed a DVT following repair (Van der Made et al. 2015). While this study reported on patient demographics, mechanism of injury, acute vs chronic and the complete vs partial nature of the avulsion, none of these specifics were known for the 3 patients (0.78% of 387 total) who developed a DVT post-operatively (Van der Made et al. 2015). Similarly in 2011, Harris et al. conducted a systematic review of outcomes in patients following repair of proximal hamstring tendon avulsions (Harris et al. 2011). Of 286 patients that were treated surgically, the authors identified 2 (0.70%) instances of DVT. No data was reported regarding the nature (partial vs complete) or chronicity of the tear (Harris et al. 2011). Our study characterizes several of the factors related to the development and treatment of DVTs following proximal hamstring repair, providing more context to contribute to the literature. These include DVT prophylaxis as well as method and duration of DVT treatment. Some of the studies in our review did not include certain demographic data for the patients who developed a DVT postoperatively. The lack of information leaves a gap in knowledge regarding DVTs. To decrease the incidence of DVTs in patients following a proximal hamstring repair, possible risk factors must be identified in all cases to find a potential cause as to why this postoperative outcome occurs.
In 2021, Reza et al. performed a systematic review of 27 studies published between 2000 and 2019 to examine outcomes following proximal hamstring repair (Reza et al. 2021). Thirty different measurement methods were identified that included the postoperative results of 1,080 patients. 17 (56.7%) were patient-reported outcome measures (PROMs), such as patient surveys and Perth Hamstring Assessment Tool (PHAT), and 13 (43.3%) were clinical outcome measures (COMs), including return to sports and isokinetic hamstring strength using dynamometer. This study reported that most of the observed studies used more COMs than PROMS, and the authors concluded that a combination of COMs and PROMs was the most reliable method to test postoperative outcomes after proximal hamstring repair (Reza et al. 2021). In contrast to this study, our main focus was the occurrence of postoperative DVT; however, some of the studies in our review did mention the use of subjective methods from patients to measure postoperative outcomes.
Engler et al. conducted a retrospective cohort study in 2019 examining the incidence of DVT in the setting of proximal hamstring repair (Engler, Bragg, and Miller 2019). Of 132 patients who underwent surgical repair of an acute, complete tear, 10 (7.5%) developed DVTs. Of these, 5 (50%) were diagnosed preoperatively, and all 5 (100%) occurred in patients who were not receiving DVT prophylaxis. The remaining 5 DVTs (50%) were diagnosed postoperatively, all 5 (100%) were in patients who were receiving either aspirin or enoxaparin for prophylaxis. Of the 10 DVTs, 6 (60%) were later found to have an identifiable risk factor, ranging from Factor V Leiden, smoking, contraceptives, a long plane flight, as well as varying degrees of preoperative and postoperative immobilization. With respect to immobilization, all patients were toe-touch weight-bearing for two weeks, with progression to partial then full weight-bearing at 4 weeks. All patients used a hip brace (except for 1 knee brace) for the same duration. Of note, among the 52 partial hamstring ruptures that were treated surgically, none developed a DVT (Engler, Bragg, and Miller 2019). Engler displayed a similar degree of detail regarding the partial vs complete nature of the injury as well as the prophylaxis received by patients, though it only describes acute injuries treated by a single surgeon, with all patients receiving similar postoperative care. Overall, this review identified several factors concerning DVT complication, including partial vs complete and acute vs chronic nature of the injury in patients who developed a DVT following repair of a proximal hamstring avulsion. Though these characteristics have been evaluated in previous studies, our study included other considerations regarding DVTs, such as prophylaxis and treatment methods.
Limitations
There are several limitations to this study. First, 39 of the 47 studies (82.9%) which discussed complications in the setting of proximal hamstring repair did not indicate whether any patients developed a DVT post-operatively. That could be for a variety of reasons, including that no patients developed a DVT or no DVT data was collected. Since neither of these instances are specifically mentioned, the actual incidence of DVT in these procedures may be higher or lower than suggested by the 8 included studies due to the lack of DVT data in the other 39 articles. Additionally, information such as age, patient sex, number of tendons involved, and time to surgery following injury was not specified for the patients who developed a DVT in most of the included studies. This made it difficult to discern correlations between patient demographics, the nature of their injury, and ultimately the presence or absence of a DVT during their postoperative course. Further, details regarding the type and duration of anticoagulation given to patients with DVT were not specified throughout all the studies examined, limiting further analyses between them. Finally, a meta-analysis could not be performed for our study due to the heterogeneity of data included in the studies. All studies included in this systematic review were level III or IV, excluding them from eligibility for meta-analysis.
Conclusion
Currently, there is limited data available regarding the incidence of DVT following repair of proximal hamstring avulsion injuries, as well as a lack of consistent protocols for post-operative DVT prophylaxis. Most DVTs occurred in patients with a complete proximal hamstring tendon avulsion. Orthopaedic surgeons should understand that patients undergoing repair of proximal hamstring avulsion injuries are at risk of developing a DVT and surgeons should, therefore, consider putting these patients on DVT prophylaxis postoperatively. Further studies are needed to clearly define the rate of DVT following proximal hamstring repair and the appropriate protocol for DVT prophylaxis.