Introduction
Restoration of function and prevention of complications through the healing of created defects are the ultimate clinical goals following the excision of benign bone tumors. Benign bone tumors are commonly treated and managed by curettage and grafting with either autogenous bone graft or synthetic bone filler (Harimtepathip et al. 2021). Autogenous bone grafts have been considered the gold standard in treating these benign lesions as they contain the necessary properties required for bone osteoinduction, osteoconduction, and osteogenesis (Wang and Yeung 2017). However, there are various limiting factors to autogenous bone grafts, including limited volume availability, increased risk of infection, and donor site morbidity (Pape, Evans, and Kobbe 2010; Mihai-Aurel, Andrei, Bogdan, et al. 2021). Allogenic bone grafts have also been considered as alternatives to autogenous bone grafts, although they may be immunogenic and have demonstrated higher failure rate in one series (Wang and Yeung 2017).
The limitations and risk factors associated with both autogenous and allogenic bone grafts have led to the development of a variety of synthetic bone substitutes to fill the defects created after curettage of benign bone tumors (Kotrych, Korecki, Ziętek, et al. 2018). Synthetic bone substitutes are commonly derived from bio-ceramics such as calcium phosphates and calcium sulphate (Kotrych, Korecki, Ziętek, et al. 2018).
CERAMENTTM Bone Void Filler is one of many synthetic bone substitute materials shown to promote cancellous bone healing and reproduce remodeling in bone defects (Abramo et al. 2010). The synthetic bone graft substitute (BGS) is injectable and moldable, composed of 40% hydroxyapatite (HA), 60% calcium sulfate (CS), and the radio-contrast agent iohexol (Hofmann, Gorbulev, Guehring, et al. 2020; Hettwer, Horstmann, Bischoff, et al. 2019; Kaczmarczyk et al. 2015). This combination is designed to resorb at a rate that matches bone formation, with CS serving as a fast resorbing material and the highly osteoconductive HA providing the scaffolding for guiding bone cells to grow in and onto the bone graft material, promoting bone ingrowth, making it suitable for both minimally invasive and open surgical procedures (Hofmann, Gorbulev, Guehring, et al. 2020; Hettwer, Horstmann, Bischoff, et al. 2019; Kaczmarczyk et al. 2015; Nilsson, Zheng, and Tägil 2013). CERAMENT used in this study had no intrinsic osteoinductive properties. This study aims to evaluate the BGS safety profile, its effectiveness in facilitating bone repair, and the degree to which it integrates with the natural bone tissue as observed in radiographic studies. The primary objectives of this study include:
- Measure radiographic patterns and serial extent of bone resorption and incorporation of CERAMENT,
- Evaluate rate and timing of post-operative functional recovery,
- Evaluate rate and timing of postoperative complications.
The hypothesis guiding this study is that CERAMENT will demonstrate a favorable safety and efficacy profile in the clinical setting following curettage and grafting of benign bone tumors. We specifically anticipated observing progressive and balanced resorption and incorporation into the bone, thereby promoting effective bone healing over the duration of the study period with minimal complications secondary to the synthetic bone material.
Materials and Methods
A retrospective chart review of 81 curettage and grafting procedures for benign bone tumors with placement of CERAMENT in 78 patients by the senior author between 2016-2023 was performed. The senior author chose to use this filler based upon demonstrably prolonged incorporation duration with other fillers (Damron and Mann 2018; Damron 2007; Damron et al. 2013). CERAMENT-G, a gentamycin-containing synthetic bone graft substitute, was not utilized by the senior author. Indications for surgery included aggressive bone tumors, unexplained pain, associated concurrent pathologic fracture, history of fracture through the lesion, indeterminate diagnosis, and impending fracture. Pre-operative imaging modalities, including X-ray, magnetic resonance imaging (MRI), and computed tomography (CT) were used to establish the location, type, and dimensions of each osseous lesion. MRIs were the preferred preoperative imaging modality for surgical planning purposes as they are considered the best tool for characterizing the extent of bone marrow and soft tissue involvement (Goyal et al. 2019). Lesional tissue was evaluated by pathology in all cases. The clinical practice of the senior author was to obtain intraoperative frozen section margins for tumors with aggressive or indeterminant preoperative features and to send tissue for all others post-operation for permanent pathology. Operative notes provided technical details, bone defect dimensions, and volume of the BGS used to fill the defect. Routine post-operative surveillance follow-up consisted of clinical evaluation and plain radiographs at 6-weeks, 3-months, 6-months, 1-year, and 2-years to monitor the site for any signs of complications such as infection, displacement, or rejection. Post-operative plain radiographs were analyzed by two radiologists to evaluate the rate and quality of graft incorporation, as well as the resorption patterns of the bone filler, utilizing the modified Neer classification (Table 1) (Wu, Chen, Chen, et al. 2018). Plain radiographs were used as they are typically the most reliable imaging modality to identify areas of bone resorption and to avoid the cost of MRI and increased radiation exposure of CT (Thippeswamy et al. 2021). The puddle and halo signs, as well as graft within the soft tissue, were common manifestations identified on post-operative radiographs. Puddling refers to a hyperdense accumulation of graft material within the interosseous gravity-dependent portion of the defect (Ferguson et al. 2019). Halo sign refers to a peripheral radiopaque ring at the interface between the healing bone and the incompletely incorporated BGS (Ferguson et al. 2019). Graft within the soft tissue refers to radiographic evidence of extraosseous BGS leaking outside of the created defect. Primary post-operative complications sought in the chart review included superficial and deep wound infection, nerve injury, graft leakage and prolonged drainage, tumor recurrence, pathologic fracture, and need for reoperation. This study was approved by the Institutional Review Board.
The treatment group consisted of 47 male and 31 female patients with a total mean age of 25.2 (range, 2 to 69). In the case of the 2-year-old patient, bone void filler was utilized due to the substantial bone loss following curettage of a large (~65 cm³) aneurysmal bone cyst in the left scapula. While the patient’s young age and natural reparative capacity were considered, the senior author felt the extent of the defect warranted an osteoconductive filler to facilitate bone healing. Patients were followed for up to 2 years. Location of the benign lesions included 21 femurs, 15 tibias, 14 humerus’, 9 fibulas, 5 radius’, 5 feet or metatarsals, 4 hips, 3 hands, 2 clavicles, one each ulna, patella, and scapula. Campanacci grade was applied to each lesion by the senior author through preoperative imaging and included 47 latent, 25 active, and 8 aggressive bone lesions (Mavrogenis et al. 2017). The lesions identified by pathology included 22 unicameral bone cysts (UBC), 12 enchondromas, 11 aneurysmal bone cysts (ABC), 7 non-ossifying fibromas, 5 due to fibrous dysplasia, 4 intraosseous ganglia (IO), among others (Figure 1). Simple curettage was utilized by the senior author for 67 procedures, whereas extended intralesional curettage was used for more aggressive tumors, including 11 ABCs, 2 GCTs, 1 chondroblastoma, and 1 chondromyxoid fibroma.
Preoperative imaging was used to measure the lesional dimensions in all cases. Surgically created defect dimensions were obtained from intraoperative measurements recorded in the operative report. Both measurements were available in 61 patients. Preoperative lesion dimensions were converted to volume by multiplying the length x width x depth of each lesion through MRI and CT scans, recognizing that this is an oversimplification of their actual complex dimensions. The average preoperative lesion volume was 19.8 cm3 (median, 8.4 cm3; range, 3.7 to 148 cm3, n=78), and the average surgically created defect volume was 18.3 cm3 (median, 6.8 cm3; range, 0.19 to 132.0 cm3, n=61). The average volume of the BGS used was 13.2 cm3 (median, 10 cm3; range, 1 to 80 cm3). Discrepancies in dimensions of preoperative lesion and curettage defect size stem from differences in measurements techniques; preoperative lesion size was measured precisely through imaging whereas curettage defect size was measured using a ruler in the operating room and were often difficult to accurately assess due to limited access through the created cortical access portal. Eleven patients were treated for preoperative fracture secondary to the benign lesion with concurrent open reduction and internal fixation. Fifteen patients were treated with prophylactic stabilization during the procedure. The need for prophylactic stabilization was determined by the senior author for those with an elevated risk of post-operative fracture, considering factors including the size and location of the defect, age and comorbidities of the patient, and estimated post-operative activity level. The remaining 52 patients were treated for pain attributed to the identified bone lesions.
Results
The mean follow-up was 12.6 months (range, 2 weeks to 28 months). Follow-up time periods were designated as 6-weeks (90.1% patient follow-up rate), 3-months (70.4%), 6-months (63.0%), 1-year (44.4%), and 2-years (30.9%).
3.1. Patterns and extent of the BGS resorption and incorporation
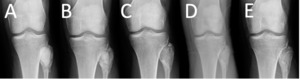
The healing of bone demonstrated a centripetal progression of graft resorption and incorporation through a zone of peripheral radiolucency. Partial but progressive graft resorption and incorporation with bone healing was evident at 6-weeks and 3-months in 43.8% and 54.4% of patients, respectively, with frequent puddling in 27.4% at 6-weeks and 29.8% at 3-months (Table 2). Advanced or complete resorption and incorporation was demonstrated at 6-months (72.5%), 1-year (80.6), and 2-years (84.0%) with puddling evident in 12% of patients at 2-years (Figure 2 & 3). Radiographic evidence of filler in the soft tissues was noted in 6.8% of patients at 6-weeks and 5.3% of patients at 3-months but resolved over time without clinical manifestation in this study population.
3.2. Rate and timing of functional recovery
Weight-bearing status was assessed at each follow-up, with 88% of patients achieving full weight-bearing status by three months. This included 100% of patients with upper extremity surgeries and 84.6% of those with lower extremity surgeries.
3.3. Rate and timing of complications
3.3.1. Recurrences
There were 5 recurrences (3 UBCs, 2 ABCs), 3 of which required reoperation. One patient required two procedures for two separate recurrences of a UBC of the left femur that were observed at 8 and 18 months, respectively, and both were re-operated on within one month of detection. After the second reoperation, the patient was found to have complete graft incorporation at 1-year with no further complications. The other reoperation was for a patient who initially presented with a UBC of the right humerus, with reoperation resulting in complete graft incorporation at the 1-year follow-up without further complications. Another recurrence was noted one year post-operation, with reoperation postponed for another year to allow for skeletal maturity. The remaining two recurrences occurred at 21 months and 1 year, with no secondary procedures required.
3.3.2. Fracture
One fracture through the grafted lesion of a UBC within the left humerus occurred one-year post-operation that did not require a secondary procedure. This complication could conceivably have been attributable to slow incorporation of the graft material leading to persistence of the defect as a stress riser that predisposed to fracture with the appropriate injury. In this case, the precipitating injury was a fall onto an outstretched hand. At the follow-up time points from 6-weeks to the time of injury at 1-year, radiographs done for this patient determined a modified Neer score of 3 at each time-point, identifying slow incorporation of the BGS, which could conceivably make us believe the fracture likely occurred secondary to a precipitating injury of a fall onto an outstretched hand that led to the fracture of a weakened bone.
3.3.3. Infection, Neuropathy, and Limited Mobility
Four patients were treated with antibiotics for local infections 6-week follow-up. Three other complications resolved completely without secondary treatment. These complications included motor neuropathy and paresthesia of the operated limb and limited joint mobility near operated site. The patient with motor neuropathy and paresthesia of the thigh underwent surgery for a UBC of the left acetabulum. This patient experienced femoral motor neuropathy at the 6-week follow-up, resulting in leg extension weakness. This condition resolved without further treatment by the 3-month follow-up. The patient with limited joint mobility underwent surgery for an IO in the head of the right fibula. The patient experienced limited mobility at the 6-week follow-up time point which was determined to be secondary to increased usage of a wheelchair. The patient was encouraged to increase mobility and resolved at the 3-month time-point.
There were no complications directly associated with the graft material, such as spitting out and prolonged drainage of graft material. Spitting out is defined as visible granules of graft material exuding through the operative incision, as reported previously in the literature (Lee et al. 2015). Prolonged serosanguinous drainage and wound weeping occurred in the four patients with infection, which resolved following antibiotic treatment, and were not related to complications of the graft material itself.
Discussion
This study highlights the safety and efficacy of CERAMENT following the curettage of benign bone tumors, demonstrating promising results and supporting the initial hypothesis that the BGS is safe and effective for clinical use in this context. Advanced or complete resorption and incorporation was demonstrated in 73.0% of lesions by 6-months, functional restoration was concordant with what would be expected after curettage of these benign bone lesions, and there were no graft-related complications.
Historically, treatment of benign bone tumors indicated for operative management has included intralesional curettage and bone grafting in order to restore structural integrity and functional stability of the bone (Dong et al. 2020). Autogenous bone grafts, while considered the gold standard, come with limitations of donor site morbidity and limited volume availability (Pape, Evans, and Kobbe 2010; Mihai-Aurel, Andrei, Bogdan, et al. 2021). Allogenic grafts, too, present challenges, primarily due to their immunogenic nature and higher failure rates (Wang and Yeung 2017). Allografts also carry a very small risk of disease transmission (Zamborsky et al. 2016; Coraça-Huber, Steixner, Najman, et al. 2022). Synthetic grafts have been developed as an alternative solution to these conventional fillers.
The effectiveness and safety profile of the BGS noted in this study indicates its potential as an alternative filler, eliminating risks associated with donor site morbidity, graft rejection and disease transmission. Our findings are consistent with and further substantiated by earlier studies that also document the safety and efficacy of this BGS for filling defects created by curettage of benign bone tumors (Table 3). Notably, Kotrych et al. (2018) reported significant pain reduction by decreased VAS scores and functional improvement by improved MSTS scores in 33 patients over a 13-month follow-up period (Kotrych, Korecki, Ziętek, et al. 2018). Similarly, Dong et al. (2020) observed complete resorption and minimal complications, coupled with a low recurrence rate two years post-treatment in 17 patients treated with the BGS (Schaser et al. 2002). Kaczmarczyk et al. (2015) followed 14 patients for 12 months after treatment and noted immediate pain relief, rapid recovery to weight-bearing, and effective bone consolidation and incorporation (Kaczmarczyk et al. 2015). Horstmann et al. (2018) followed 35 patients for 12 months after open curettage and observed normalized cortical thickness on radiographs in 79% of cases at 1-year noting 4 patients with a recurrence of their bone cyst (Horstmann et al. 2018). These studies collectively affirm the findings of our research, demonstrating the BGS’s consistent performance across multiple studies, thus underlining its reliability as a filler following curettage of benign bone tumors.
While synthetic bone void fillers like CERAMENT provide clinical advantages, they are associated with a relatively higher cost and a risk of local reactions, especially with calcium sulfate-containing grafts (Roberts and Rosenbaum 2012; Lun, Li, and Li 2024). These factors should be considered when weighing the benefits and risks of using synthetic bone void fillers for bone defect reconstruction.
The pattern of graft resorption and incorporation observed in this study reflects a balanced and progressive process, contributing to the long-term success of bone defect healing. The modified Neer classification provided a detailed analysis of healing stages, showing significant progress by the six-month follow-up and near-complete integration in most patients by two years. These observations suggest that the BGS’s composition of hydroxyapatite and calcium sulfate supports both initial stability and gradual integration with host bone tissue.
Our study stands out for tracking the largest cohort of defects filled with CERAMENT over the longest follow-up time period reported to date. The long-term data we gathered demonstrates that the BGS achieves high rates of graft resorption and bone healing with a low complication rate attributable to the graft. The progression of full weight-bearing status by three months post-operation in 88% of patients underscores the effectiveness of the BGS in rapidly restoring function. Notably, our study observed no cases of spitting out of the graft material, prolonged drainage, or pathological fractures specifically due to the recurrence of cysts during the healing phase, underscoring the reliability and safety of this BGS in clinical use.
Limitations
The non-comparative and non-blinded design of this single-center retrospective chart review limits the generalizability of the findings. Selection bias, particularly regarding patient characteristics such as age and bone healing capacity, is an inherent risk. Additionally, patient drop-off over the study’s duration reduced the sample size for long-term follow-up, which may decrease the statistical power to detect subtle differences or complications, particularly within subgroups. Future prospective, multicenter studies with randomization and blinding are necessary to confirm our findings and further validate the use of synthetic bone grafts in treating benign bone tumors.
Conclusion
This study demonstrates that CERAMENT is an effective and safe option for the reconstruction of bone defects following the excision of benign bone tumors. Our findings establish that this BGS facilitates rapid functional recovery and bone integration with a favorable safety profile, underscoring its potential as an attractive alternative to traditional bone grafting methods in orthopedic surgery.