INTRODUCTION
Acromioclavicular (AC) separation is a common injury of the shoulder girdle accounting for more than 40% of all shoulder injuries (Kiel, Taqi, and Kaiser 2022). The most common cause for AC joint separation is a fall directly onto the shoulder, with a downward force imparted on the superior aspect of the acromion. This in part depresses the scapulohumeral complex and causes disruption of the AC ligament and the coracoclavicular (CC) ligaments (van Bergen et al. 2017). Injuries of the AC joint are often classified by the Rockwood classification. The Rockwood classification takes into account integrity of the AC and CC ligaments, AC joint disruption, and deltoid and trapezius muscular attachments. Type I and II are generally treated nonoperatively while types IV – VI are generally treated operatively leaving type III injuries to be treated on a case-by-case basis (van Bergen et al. 2017; Martetschläger et al. 2019).
Numerous surgical techniques have been employed in the treatment of AC separations, but complications are still common and can include migration or loosening of hardware, loss of reduction, nerve injury, graft rejection, arthritis, continued pain and stiffness, and cosmetic deformities, with reported rates ranging from (5-20%) (Berthold et al. 2022) . The complication rates and associated morbidity, even with modern fixation methods, led Dr. Richard Hawkins to comment “any reconstruction of the AC joint is looking to fail” (Hawkins, n.d.). Historically, hook plate fixation has been used in the treatment of acute AC separation due to ease of technique, reliable stabilization, and minimal need for additional resources (G. Liu et al. 2022). Hook plate fixation is also suitable for use in chronic AC separations, but often necessitates use of allograft or autograft augmentation to restore the AC and CC ligaments (C. T. Liu and Yang 2020; Salem and Schmelz 2009). Regardless, removal of the hook plate is recommended at approximately 3-4 months from the index procedure to prevent hardware irritation and potential acromial osteolysis.
The BioBrace® implant is a bioresorbable, porous, Type 1 collagen matrix reinforced with PLLA fibers that is used to provide initial strength and encourage healing of torn tendon/ligament tissue. Approved by the FDA in 2021, the implant has been used in numerous soft tissue reinforcement techniques including rotator cuff repair, ACL reconstruction, and Achilles tendon repair. In this case report, we describe the fixation of a chronic traumatic AC separation using a hook plate and a 5mm x 250mm collagen strip of BioBrace to reconstruct the AC and CC ligaments. In addition, we present histological analysis of the collagen implant 3 months after implantation.
CASE PRESENTATION
A 52-year-old male sustained a traumatic workplace injury in which he was assaulted by a coworker and thrown to the ground, resulting in pain and deformity to the right shoulder. Initial X-Rays indicated the presence of an AC joint separation with approximately 1.5x shaft posterior superior displacement of the distal clavicle with respect to the acromion. Widening of the coracoclavicular interval was present, suggesting injury of the ligaments as well (Figure 1). MRI was obtained and demonstrated disruption of the CC ligaments and AC joint capsule. Effusion was present in the disrupted AC joint and edema along the course of the CC ligaments (Figure 2).
PREOPERATIVE PLAN
Options for care were discussed with the patient. Based on his presentation with continued pain, deformity, and work demands, recommendations were made for operative treatment of AC/CC reconstruction using a hook plate and BioBrace® augmentation, followed by subsequent hardware removal approximately 3 months post initial procedure.
OPERATIVE TECHNIQUE
The patient had a supraclavicular nerve block and was positioned in a 45 degree beach chair position. Examination under anesthesia revealed a fixed and irreducible prominence at the right AC joint. A six-centimeter transverse incision was established in line with the distal clavicle and centered over the AC complex. Skin flaps were elevated, and blunt dissection was performed down to the AC joint through the traumatic rent in the deltotrapezial fascia. The distal aspect of the clavicle was medially exposed using a periosteal elevator to gently free soft tissue. The ligaments of the AC joint were confirmed to be disrupted.
A minimal osteotomy was performed using rongeur and oscillating saw along the distal clavicle in order to remove osteophytes and facilitate reduction. The clavicle was manually reduced in line with the acromion to prepare for joint reconstruction. A k-wire directed from acromion into the clavicle shaft was used to maintain provisional reduction.
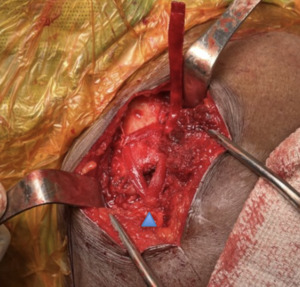
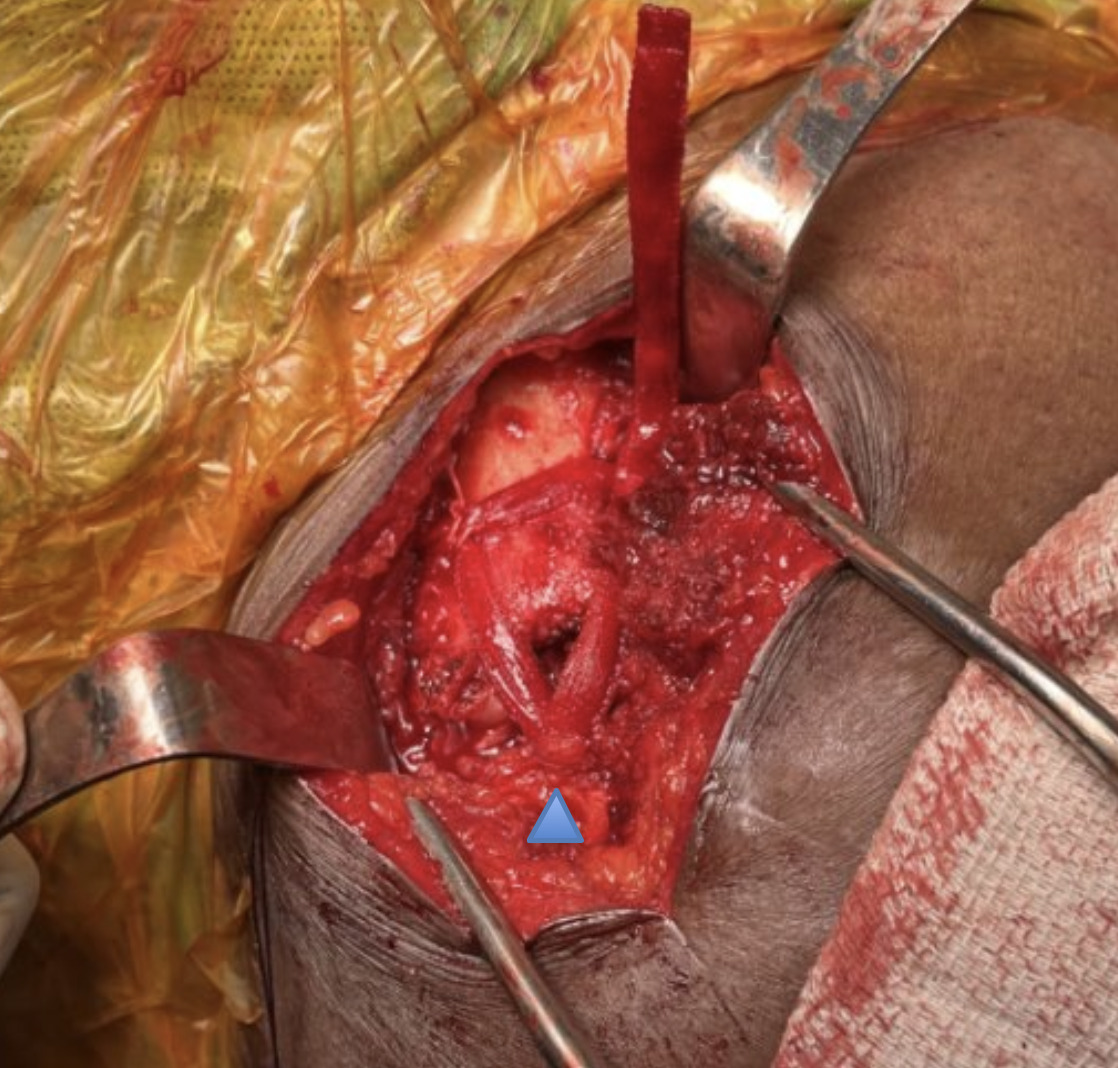
A blunt Haney needle driver was introduced underneath the inferior aspect of the base of the coracoid from medial to lateral. Alternatively, a cerclage passer or other blunt curved instrument may be used. A needle driver was used to grasp a looped suture to pass suture from lateral to medial around the undersurface of the coracoid. A 5 mm x 250 mm BioBrace® was pre-soaked in approximately 20 cc of whole blood and passed postero-laterally over the clavicle and then passed underneath the coracoid process from medial to lateral using the previously placed looped suture as a shuttle. The implant was then brought anteriorly to the clavicle forming a continuous loop, recreating the approximate course of the trapezoid and conoid portions of the coracoclavicular ligaments. The BioBrace® implant was then clamped with a hemostat to provide provisional reduction of the clavicle. Several interrupted nonabsorbable sutures were then tied to secure the implant to itself over the clavicle. Knots were buried and cut flush to avoid knot prominence. The BioBrace® was then brought along the posterior superior surface of the clavicle. The lateral end of the BioBrace® was positioned laterally over the AC joint and sutured to the remnants of the AC superior capsule to provide provisional reduction and reconstruction of the AC joint. The remaining free tail of the BioBrace® strip was secured with a hemostat to maintain the reduction until plate fixation was complete.
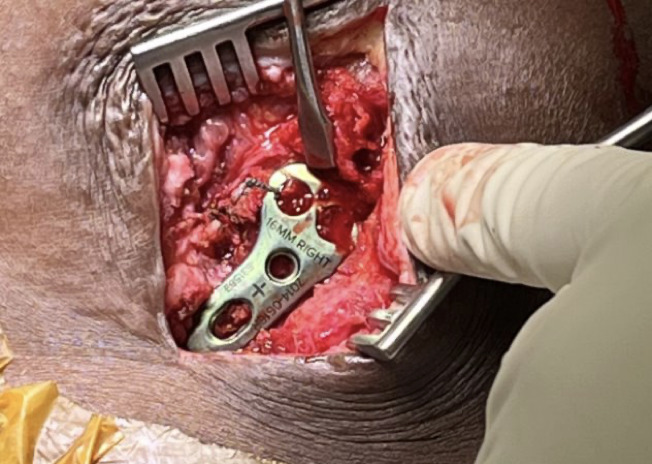
A hook plate depth sizer guide (Acumed) was provisionally secured with a plate tack over the clavicle and into the AC joint. The 16 mm size was selected based on fluoroscopy confirmation. Definitive 5- hole hook plate was then placed over the clavicle over the provisionally secured BioBrace® implant. Hook plate was fixated to the clavicle with 3 clavicle locking screws in standard fashion ensuring there was no violation of the Biobrace implant during insertion. The BioBrace® was then further assessed to ensure that appropriate reduction of the AC joint and the CC was achieved.
Fluoroscopic images were obtained to ensure optimal plate position, AC joint reduction, and no evidence of instability at the glenohumeral joint or impingement with abduction. The remaining length of the BioBrace® implant was passed along the anterior superior aspect of the AC joint and secured to the anterior “trapezoid” limb of the BioBrace® reconstructed ligament with several #0 Vicryl sutures (Figure 5). A remnant tail of the BioBrace® was then secured to the anterior hole of the hook plate with a nonabsorbable suture for later identification and biopsy harvest during planned hook plate removal at 3 months.
The deltotrapezial fascia was reapproximated and closed with #1 Vicryl using a “pants over vest” technique, followed by subcuticular skin closure with 3-0 Monocryl and Steri-Strips. The patient followed a standard AC joint post-operative protocol utilizing a simple sling, and avoidance of overhead activities. Range of motion exercises were initiated at week 6 with the goal of restoration of full range of motion by week 12. Strength exercises were avoided to allow for adequate healing. The patient returned to the office at 2, 6, and 12 week postoperative time points for evaluation.
At approximately 3 months, the patient returned to the OR for planned hook plate removal. Intraoperative fluoroscopy with dynamic stress views were obtained and demonstrated stable, anatomic alignment of restoration of the AC joint and CC interval. During the procedure, a portion of the BioBrace® implant that was previously marked with nonabsorbable suture to the anterior aspect of the plate was resected using a fresh scalpel approximately 5 mm distal to the hook plate. The previously placed BioBrace® implant was intact and overlying the distal end of the clavicle and noted to have incorporation to the surrounding soft tissue. There was a congruent reduction of the AC joint with no subsidence which was stable to palpation. The wounds were thoroughly irrigated, and closure of the wounds was performed.
The hook plate with a portion of the BioBrace® implant and a sample of tissue from the surrounding area were placed in a formalin container for later histologic analysis.
POSTOPERATIVE RESULTS
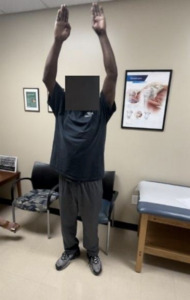
The patient was seen approximately one week after the hook plate removal and reported decreased pain with range of motion. Three weeks after the hook plate removal, the patient reported a Visual Analog Score (VAS) of 0 and no cosmesis concerns. Radiographs obtained at this visit showed maintenance of AC reduction. At 3 months post-op from the plate removal, the patient had returned to light duty and demonstrated full range of motion, a VAS of 0, and a Single Assessment Numeric Evaluation (SANE) score of 95% (Figure 10). Radiographs obtained at three months demonstrated no loss of AC or CC alignment (Figures 11 and 12). Ultrasound evaluation at this visit demonstrated the BioBrace® implant along the superior clavicle with reconstruction of the AC capsule. No fluid levels or calcific deposits were appreciated within the surrounding tissue or capsule (Figure 13). At 6 months post-op, the patient was released to full duty with no restrictions and returned to prior level of function and demands as a forklift operator. Patient was subsequently released from our clinic at 9 months and reported no issues with work demands and a final SANE score of 97%.
HISTOLOGY
Materials and Methods
Histological Evaluations
To determine the ability of the BioBrace® (CONMED Corporation, Largo, FL) implant to support local cell infiltration and tissue integration, qualitative histological assessments were performed. The explanted BioBrace® implant and associated soft tissue was fixed in 10% neutral buffered formalin for 48 hours prior to undergoing routine histological processing and paraffin embedding. The sample was oriented and sectioned at a thickness of 5 µm using a rotary microtome (Leica) resulting in visualization of an axial cross-section of the implant. Sections were deparaffinized and rehydrated prior to being stained with hematoxylin and eosin (H&E; n=3) to visualize extracellular matrix (ECM) and cell nuclei, as well as Masson’s Trichrome (MT; n=3) and Picrosirius Red (PR; n=3) to visualize collagen and collagen alignment, respectively. A composite image of the entire implant cross-section was stitched together and representative images from different regions within and around the implant were captured using a Keyence BZX 800 microscope.
Results
After 12 weeks of implantation, polymer fibers of the BioBrace® were evident within all histological sections as indicated by unstained / translucent circular fiber cross-sections. H&E staining illustrated cell infiltration and ECM deposition within and around the implant fibers (Fig. 14A). Morphology of cell nuclei indicated a mix of inflammatory cell (indicated by round nuclei) and fibroblast (indicated by elongated nuclei) infiltration within the implant (Fig. 14. B&C; open triangles). Additionally, multinucleated foreign body giant cells were noted adjacent to the polymer fibers of the implant (Fig. 14. B&C; filled triangles). The presence of sparse blood vessels and red blood cells were also present throughout the implant. MT staining illustrated that much of the ECM deposited around and within the implant was primarily composed of collagen (i.e. blue staining). Evidence of a collagenous capsule was found surrounding the implant (Fig. 15A) and collagen fibers were found infiltrating within the implant interstice and between its polymer fibers (Fig. 15. B&C). PR staining confirmed the deposition of collagen around and within the BioBrace® implant (Fig. 16. A-C).
The summary of the histologic analysis at the time of explant was that new cells and tissues were infiltrating from the periphery and proliferating within the BioBrace® implant and it was actively being remodeled. Foreign body giant cells appeared to be degrading the residual polymer fibers of the implant and fibroblasts were depositing collagen within and around the implant. An inflammatory response was also present; however, this was not an unanticipated finding as the implant will be degraded and replaced by tissue over time.
DISCUSSION
This case presents the first described method of using a reinforced implant, BioBrace®, as an augment for CC ligament reconstruction in the setting of a chronic AC joint dislocation. In addition, this is the first report of BioBrace® tissue explanted from a human with corresponding histologic evaluation. Analysis reveals favorable host tissue incorporation and graft remodeling consistent with prior animal retrieval studies (Walsh et al., n.d.; Van Kampen et al. 2013).
Injuries to the AC joint can result in limited range of motion and decreased shoulder function. Strategies for nonsurgical management of AC joint injuries have been described as early as the days of Hippocrates and Galen (G. Liu et al. 2022). In the modern area, numerous surgical techniques, both open and arthroscopic, have been implemented, but complications are still common despite these advances. Introduced by Balser in 1976, the hook plate technique continues to have worldwide use (Jeong and Chun 2020). The hook plate technique uses a contoured plate of varying sizes that mimics the natural anatomy and articulation in the AC joint to maintain reduction. The plate is fixed using screws to the superior surface of the distal clavicle and hooks under the undersurface of the acromion and works as an internal splint to reduce the AC joint while the injured CC ligaments heal (Kumar and Sharma 2015). Plate removal is recommended to reduce risks from complications due to acromial osteolysis, subacromial impingement syndrome, loosening of internal fixation and clavicle stress fracture (Stucken and Cohen 2015). Manufacturers recommend hook plate removal after 3 months, or once the CC ligaments have healed.
Failure of the CC ligaments to heal is a concern during the recovery process and augmentation remains controversial. Some surgeons elect to perform hook plate fixation without CC augmentation citing an increased operative time, increased costs, and a larger surgical wound. Studies report that CC ligament augmentation significantly enhances the stability and function of the ligament complex, improves short term outcomes, and decreases complication rates (C. T. Liu and Yang 2020; Salem and Schmelz 2009; Chen, Wu, Jhan, et al. 2021a; Motamedi et al. 2000). Combined hook plate and CC reconstruction using a nonabsorbable Mersilene loop suspensor device has previously demonstrated to have fewer acromial osteolysis complications and improved maintenance of reduction (Chen, Wu, Jhan, et al. 2021b). In the current technique, we sought to replace the use of a nonabsorbable synthetic Mersilene tape with a similar construct using a novel absorbable, reinforced collagen implant.
BioBrace® is FDA approved to provide reinforcement to healing tissue and was utilized as augmentation in this technique. It is a highly porous type-1 collagen matrix with reinforced PLLA microfilaments. The implant provides strength at time zero by load sharing during the healing process with complete resorption at approximately two years (Carter et al. 2021). The implant is a unique biocomposite reinforcement that provides strength and promotes healing. BioBrace was used to augment and reconstruct the chronic AC and CC ligament injuries. This was achieved by passing the implant in a near-anatomic position to effectively recreate the trapezoid and conoid limbs of the AC joint. This provided a biologic scaffold to promote healing of the ligaments. Although a hook plate was used in this case for initial mechanical fixation, other methods of initial mechanical fixation could be considered, such as suspension suturing or anchor fixation between the coracoid and clavicle.
LIMITATIONS
The case presented demonstrated a favorable result based on patient reported measures, radiographic, ultrasound, and histologic analysis. Confounders to the success of the case are the concomitant use of a hook plate for fixation and the reinforced collagen implant for reconstruction of the AC/CC complex. Future biomechanical investigations would be ideal to determine if the reinforced collagen implant construct used to recreate the AC and CC ligaments in this case could be used in isolation in the setting of acute or chronic AC dislocations, obviating the need for hook plate implantation and its subsequent removal. Further outcome studies would also be needed to determine if BioBrace® augmentation offers improved patient benefit for the treatment of AC dislocations.
CONCLUSIONS
AC reconstruction surgery remains challenging and methods for better healing and remodeling of the damaged ligaments and capsule are needed. This case presents a novel technique with histologic analysis in which a reinforced collagen implant was used with a hook plate to heal a chronic type 5 AC joint separation.
Acknowledgements
Histology and analysis was performed in collaboration with The South Carolina Bioengineering Center for Regeneration and Formation of Tissues (SC BioCRAFT) which is supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (Award Number: P30 GM131959) and The Laboratory of Orthopaedic Tissue Regeneration & Orthobiologics (Ortho-X) at Clemson University.












_of_the_ac_joint_at_three_months_status_post_hardware.png)
_entire_.png)
_entire_cross-section_of_the.png)
_entire_cross-section_of_the_bi.png)











_of_the_ac_joint_at_three_months_status_post_hardware.png)
_entire_.png)
_entire_cross-section_of_the.png)
_entire_cross-section_of_the_bi.png)