Introduction
Hand collateral ligament injuries, particularly at the thumb metacarpophalangeal (MCP) joint with an incidence rate of 86%, pose a substantial concern due to their potential to induce significant pain, diminished pinch strength, instability, and, if inadequately addressed, osteoarthritis (Baskies and Lee 2009; Rhee, Jones, and Kakar 2012; Samora et al. 2013). Athletes, in particular, grapple with a heightened occurrence of hand collateral ligament injuries, given the escalating physical demands of competitive sports (Lee and Carlson 2012; Johnson and Culp 2009). The pursuit of early return to play, a priority not only for elite athletes but also for weekend warriors, often necessitates ligament repair or reconstruction as the preferred course of action. However, the laudable goal of expeditious return to preinjury status encounters impediments imposed by the requisite recovery period essential for optimal ligament-to-bone healing, thereby temporarily sidelining athletic participation.
Recent trends favor the implementation of suture augmentation utilizing robust nonabsorbable constructs aligned parallel to the repair. Anchored by theoretically biologically inert polyether ether ketone (PEEK) anchors, early experiences showcase notable time-zero stability and strength in the repair, prompting several surgeons to endorse its usage for hastening return to play. For instance, De Giacomo et al reported a one-week return to practice and full unrestricted play at five weeks with nonabsorbable suture augmentation in thumb ligament injuries (De Giacomo and Shin 2017). Comparable resistance to gap formation, demonstrated in a model comparing elbow reconstruction with ligament repair using nonabsorbable suture augmentation versus autograft ligament reconstruction, was corroborated by Dugas (Dugas et al. 2016). However, potential drawbacks linger, as the protective effect of suture augmentation on the ligament might compromise the final strength and composition of the repair. Although long-term effects of stress shielding in humans remain unreported, extensive biomechanical and animal studies consistently highlight significant alterations in architecture, tensile strength, and mechanical properties. These alterations in the important role of stress and load in tendon remodeling can be significant and could hamper the healing process (Leasure et al. 2019; Yamamoto, Hayashi, and Yamamoto 2000; Majima, Yasuda, Tsuchida, et al. 2003; Uchida, Tohyama, Nagashima, et al. 2005; Viens et al. 2014). Furthermore, complications associated with nonabsorbable suture augmentation involve the use of PEEK anchors, with documented instances of osteolysis and potential weakening of the construct (Chen et al. 2023; Di Benedetto et al. 2020). The considerable size of these anchors poses the additional risk of iatrogenic or insufficiency fractures when inserted into the small bones of the hand and wrist.
In light of the potential disadvantages associated with nonabsorbable suture augmentation using large polymer anchors, there is a compelling impetus for the development of a superior ligament construct. Biological and synthetic augmentation emerges as a viable avenue, promising an accelerated recovery process by enhancing intrinsic healing mechanisms and providing load sharing to the repair or reconstruction.
Biologic enhancement, exemplified by the utilization of Type I bovine bio-inductive implants, has demonstrated success in inducing new tendon-like tissue. However, these implants lack the structural strength crucial for safeguarding the integrity of the repair. To address this shortfall, dermal allografts (acellular dermal matrices) have been recommended to impart early biomechanical strength and load-bearing capacity. Yet, their deployment introduces challenges such as delayed tissue incorporation and inherent risks associated with allograft use (Bishai et al. 2021; Bushnell et al. 2021).
A recent entrant in the realm of ligament repair, the BioBrace by Conmed, presents a novel bio-composite construct that combines bio-inductive and bioresorbable properties. Comprising a highly porous Type 1 collagen matrix derived from bovine sources for bio-induction and bioresorbable poly (L-lactide) (PLLA) microfilaments for structural integrity, the BioBrace provides an innovative scaffold, offering pull-through load sharing strength at the time of implantation (Carter et al., n.d.). This implant is absorbed over 24 months, furnishing strength to support ligament healing without the concern of permanently retaining synthetic material (Walsh et al. 2021). The advantageous features of the BioBrace create an environment theoretically conducive to optimizing ligament or graft repair, reducing early risks of native suture-tendon failure, and facilitating early rehabilitation protocols with minimized repair failure. Preliminary results in rotator cuff, anterior cruciate, and Achilles repair/reconstruction validate the BioBrace’s efficacy (McMillan, Arciero, and Ford 2021; LeVasseur et al. 2022; Redler 2022).
This report aims to introduce the novel Bio-Composite scaffold (BioBrace) and its application in the repair of collateral ligament injuries of the hand. Through an early evaluation, the report seeks to substantiate the BioBrace’s utility in fostering successful healing while enabling early return to play with minimal risk. Potential advantages, when compared to nonabsorbable suture augmentation with large space-occupying polymer anchors, will be elucidated. The discussion will encompass normal healing processes of ligamentous injuries and the potential advantages conferred by this bio-composite implant in supporting this cascade in hand injuries.
Materials and Methods
From May 2020 to September 2023, twelve high-level athletes with acute or chronic unstable ligamentous hand injuries were referred for surgery. Demographic data, injury mechanisms, radiographic findings, and MRI results were recorded. Surgical repair/reconstruction was recommended and informed consent was obtained along with the use of the BioBrace as an inductive and resorbable implant. Surgical technique is described along with subsequent evaluation of clinical and patient-reported outcomes during early follow-up. Postoperative care involved splinting for soft tissue protection, with subsequent follow-ups for assessment and transition to sport-specific braces. Outcome measures included stability, pain, range of motion (ROM), visual analog pain scores, quick DASH, and key pinch strength. Complications investigated encompassed soft tissue reactions, osteolysis, synovitis, loss of stability, infection, and reinjury. Return to play and adverse reactions, along with complications, completed the comprehensive assessment.
Results
Thirteen patients were treated with a mean age of 21 (15-49) years. Eleven (84.6%) of the cohort were high-level athletes. The mean QuickDash (QD) score was improved from 81.5 preoperatively to 4.1 by 6 weeks postoperatively. The mean Visual Analog Scale (VAS) improved from 4.3 preoperatively to 0.6 postoperatively. There have been no reported failures, infection, radiographic osteolysis or radiographic loss of alignment. Two patients developed transient
soft-tissue reactions around the surgical incision, which comprised erythema only. Both were thought to be due to undue pressure from a splint and resolved with modification. Range of motion (ROM) was non-limiting and final pinch and grip measurements were greater than 85% of the contralateral side. Among the high-level athletes, nine (81.5%) returned to sport at eight weeks postoperatively, and all had returned to sport by 12 weeks. (Table 1)
Description Surgical Technique
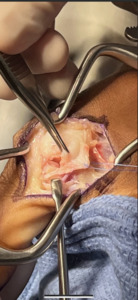
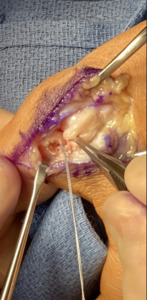
The technique described will be for repair/reconstruction of the ulnar collateral ligament (UCL) of the thumb as the majority of the cases involved this site. Slight modification is recommended depending on the site of injury. A curvilinear incision is placed on the ulnar aspect of the metacarpophalangeal joint usually 3-4 cm in length. Subcutaneous dissection identifies and protects the medial branch of the superficial radial nerve. In the case of the UCL of the thumb the adductor aponeurosis is identified and opened in an inverted “L” fashion and tagged for later closure. Specific to the injury is the presence of a Stener lesion which must be sought for and, if present, the injured ligament, usually ruptured distally, must be extracted from the interposed adductor aponeurosis and redirected to its anatomic insertional footprint. The ruptured UCL is prepared for reinsertion by drilling a pilot hole just 1-2 mm volar to the midaxial point of the base of the proximal phalanx to simulate the insertion of the proper limb of the torn ligament. The 10mm Shallow Tru-Shot Y-Knot (ConMed)(11311 Concept Blvd.
Largo, FL 33773) all suture anchor is deployed and sutured to the avulsed end of the ligament, securing it with a surgeon’s square knot. The 23x30 mm BioBrace construct is now cut to approximately a 3x1 cm format and the distal aspect secured to the previously placed suture anchor. In the majority of cases the proximal limb of the BioBrace is sutured to the proximal origin of the native UCL ligament with remaining 2-0 HiFi nonabsorbable suture. (ConMed) A supplemental transarticular k-wire is only used if compliance issues or weakened proximal UCL origin is present. If so a suture anchor can be placed 1-2 mm dorsal to the midaxial point of the metacarpal head. The adductor is repaired to its counterpart and standard closure performed. The limb is immobilized in a short arm thumb spica splint for two to three weeks at which time thumb range of motion is begun with the thumb adducted into the palm to protect the repair. At this point the thumb is started on active and passive motion with the thumb abducted followed by strengthening. Return to play is dependent on sport and position played.
Discussion
Ligamentous tears constitute a substantial proportion of hand injuries, with an even more pronounced incidence among elite athletes, impacting up to 86% of thumb injuries involving the metacarpophalangeal joint (Baskies and Lee 2009; Rhee, Jones, and Kakar 2012). The expeditious healing and timely return to play face impediments due to prolonged immobilization inherent in conservative treatments. Additionally, primary repair and reconstruction, often involving grafting, can similarly hinder recovery. Various biological and synthetic augmentation methodologies have been heralded to expedite recuperation, leveraging the native healing process. Non-absorbable implants or allografts, offering load-sharing to the repair/reconstruction, are instrumental in this pursuit.
Type I bovine bio-inductive implants have exhibited efficacy in inducing early tendon-like tissue formation, albeit with a deficiency in structural integrity. Dermal allografts, specifically acellular dermal matrices, may enhance the reparative process but suffer from protracted tissue incorporation timelines. Recent introductions, such as non-absorbable suture augmentation constructs spanning and mirroring ligament repair, have demonstrated promising time-zero stability in animal and cadaveric studies. However, concerns persist regarding potential long-term deleterious effects on the optimal healing of these injured ligaments (Leasure et al. 2019; Yamamoto, Hayashi, and Yamamoto 2000; Majima, Yasuda, Tsuchida, et al. 2003; Uchida, Tohyama, Nagashima, et al. 2005; Viens et al. 2014). These constructs rely on theoretical biologically inert anchors strategically placed in anatomically compromised locations within the hand’s bones.
The imperative to expedite return-to-play for elite athletes through non-absorbable suture augmentation, anchored speculatively, warrants scrutiny, particularly when juxtaposed with established natural biology in tendon/ligament healing. The well-described three phases of tendon/ligament healing — inflammatory, reparative, and remodeling — elucidate the necessity of applied stress in directing the alignment of collagen fibers and tenocytes, pivotal for mechanical strength optimization.
However, the long-term stress shielding effects of non-absorbable augments may impede this necessary stress application, potentially compromising the reparative and remodeling phases. Animal studies consistently underscore the adverse impact on tensile and viscoelastic properties of tendon repairs subjected to non-physiological stress shielding (Yamamoto, Hayashi, and Yamamoto 2000; Majima, Yasuda, Tsuchida, et al. 2003; Uchida, Tohyama, Nagashima, et al. 2005). Extended stress shielding adversely influences the micro and ultrastructure of tendon repairs, leading to decreased fibril formation, diameter, and an elevated inflammatory response — all detrimental factors to tendon/ligament healing.
In this context, the BioBrace emerges with distinctive advantages over non-absorbable constructs owing to its unique biological and synthetic composition. The biocomposite, featuring a porous Type 1 collagen matrix and bioresorbable PLLA microfilaments, heightens healing characteristics, providing increased strength. Preliminary evaluations of the BioBrace demonstrate its integration into injured structures within 10-12 weeks, offering sustained strength for up to two years, aligning with the targeted resorption timeline of PLLA. The unique ability of the BioBrace to reinforce, regenerate, and resorb over time constitutes a paramount advantage in creating an optimal healing milieu for injured ligaments/tendons. In this report, the first in the literature to present the results with its use in hand ligament injuries, the use of the Bio-Brace provided adequate strength to begin early range of motion, limited need for prolonged immobilization, and return to play without sequelae by 8-12 weeks. In assessing patient reported outcomes improvements in the QDash and Visual Analog Scale (VAS) support its use. There were no failures of the Bio-Brace, no bony fractures secondary to space-occupying anchors, and no patients required reoperation.
While the data available is limited, smaller space occupying all suture anchors have had preliminary evidence showing at least equivalent biomechanical properties with the advantage of having a low profile design. A small set of case series have shown little bone reaction or cyst formation around the all suture anchor, highlighting another potential benefit with the use in smaller bones of the hand (Ergün et al. 2020).
The utilization of all suture anchors to stabilize the BioBrace offers theoretical benefits, circumventing potential complications associated with large space-occupying anchors, such as iatrogenic fractures or osteolysis. This has been reported with the use of the non-absorbable brace with PEEK screws used for stabilization (Chen et al. 2023). Further, in this preliminary study encompassing 13 ligament repairs in the hand, the Bio-Brace demonstrated ease of use, minimal adverse effects, and complications. In a preliminary study encompassing 13 ligament repairs in the hand, the BioBrace demonstrated ease of use, minimal adverse effects, and complications. Noteworthy reduction in pain, distinctive decrease in swelling, and mitigated inflammatory reactions facilitated early range of motion, paralleling outcomes achieved with non-absorbable constructs. The absence of a substantial polymer anchor requirement, coupled with the eventual resorption of the bio-composite brace, underscores its utility and distinctive capability to reinforce, regenerate, and resorb over time.
Conclusion
The purpose of our preliminary study is to introduce the use of the BioBrace in the hand, describe our technique, and provide an early assessment of both clinical and functional outcomes. It is our hope that with longer term follow-up and assessment its use will be accepted into the armamentarium of these challenging injuries. Future research should investigate the impact of decreased immobilization duration on complications associated with surgical reconstructions. The potential effects may be more precisely delineated through the use of larger sample sizes targeting specific injury sites, stratified treatment arms differentiating between acute and chronic injuries,, and an emphasis on whether the injury was addressed through repair or reconstruction methods. Such studies could facilitate the development of more individualized and effective treatment strategies and rehabilitation protocols.