Introduction
More than 10 million orthopedic procedures are performed every year in the United States (AAOS Department of Clinical Quality and Value 2019). Common soft tissue procedures include repair of the rotator cuff, Achilles tendon, and ligaments of the elbow and knee (Elrod 2014; McElvany et al. 2015; Trofa et al. 2017; Bansal et al. 2021). Mechanical properties of orthopedic sutures designed to facilitate these procedures play a critical role in the success of soft tissue repair. The quality of repair can be adversely affected by utilizing materials with inadequate physical properties to support the repair construct at time zero as well as during the healing process (Najibi et al., n.d.). In particular, insufficient mechanical strength or excessive abrasion to surrounding tissue can lead to complications such as re-rupture at the site of injury, incomplete tissue repair, or overall failure of surgical repair (Açan, Hapa, and Barber 2018).
While conventional high strength orthopedic sutures are made from synthetic materials, biostimulative materials, such as collagen, have been shown to promote angiogenesis, stimulate new tissue formation, and enhance biointegration (Lee, Singla, and Lee 2001; Mazzocca et al. 2007; Enea et al. 2013; Blackstone, Gallentine, and Powell 2021). Collagen is the most abundant protein in the body, comprising approximately 30% of all proteins and serving as building blocks for numerous tissues (Shoulders and Raines 2009; Kew et al. 2011). Though collagen coated synthetic sutures (e.g., Collagen Coated FiberWire® (Arthrex, Inc., Naples, FL, AR-7200B)) are commercially available, the surface coating is intended to improve handling characteristics (Arthrex Inc. 2014). As such, there remains an unmet need for an orthopedic suture with a unique composition offering an advantageous biointegrative component while maintaining the required mechanical strength for the high physical demands of orthopedic procedures.
Studies have shown that type I collagen can be additively manufactured into fibers by extrusion (Kato et al. 1989; Dunn, Avasarala, and Zawadsky 1993) and that extruded collagen fibers can be strengthened via crosslinking to improve strength, stability, and biocompatibility (Koob and Brown, n.d.; Zeugolis, Paul, and Attenburrow 2009; Reddy, Reddy, and Jiang 2015; Suesca et al. 2017). However, producing consistent, clinical-grade collagen fiber suitable for medical device manufacture at scale has remained a challenge (Zeugolis, Paul, and Attenburrow 2009; Reddy, Reddy, and Jiang 2015; Enea et al. 2011; Delgado et al. 2015). In a previous study, we presented a method for the production of collagen fibers with high tensile strength, cytocompatibility, and low toxicity (Dasgupta et al. 2021). Briefly, a novel wet extrusion process was developed and used to produce strong, stable type I collagen fibers with diameters on the order of 150 µm. Since this study, we have further improved methodologies to produce high-strength collagen fibers at scales and costs suitable for medical device manufacturing with commercial textile processing equipment. These fibers offer immense potential as a component in medical textiles for numerous surgical applications, with one such application being biointegrative suture constructs for soft tissue repair.
The purpose of this study is to assess and compare key physical properties of novel co-braids consisting of approximately 50 wt% type I collagen fibers and 50 wt% Ultra-High Molecular Weight Polyethylene (UHMWPE) fibers to those of conventional orthopedic sutures. To ensure adequate mechanical properties for handling and maintaining tissue approximation, knotted tensile strength and knot security were determined. Knot profile and abrasiveness to tissue were also assessed as critical measures that may affect repair outcomes. In particular, suture pullout resulting from abrasion at the suture-tendon interface has remained a critical point of failure in orthopedic repairs (Kowalsky et al. 2008; Williams et al. 2016). We hypothesize that the unique composition of co-braids providing both structural and biological attributes will meet or exceed the physical properties of conventional suture while leveraging the biostimulative properties of collagen.
Materials and methods
Collagen-UHMWPE co-braid and conventional high strength suture configurations
Three commonly used orthopedic size configurations spanning a wide range of clinical applications are investigated herein for both collagen co-braids and conventional synthetic sutures: 2-0, #2, and tape. Sutures of corresponding sizes are plotted together throughout for comparison purposes. 2-0 collagen co-braid (Fig. 1) is compared to 2-0 FiberWire (Arthrex, Inc., Naples, FL, AR-7220). #2 collagen co-braid (Fig. 1) is compared to #2 FiberWire (Arthrex, Inc., Naples, FL, AR-7200). Collagen co-braid tape (Fig. 1) with a nominal width of 2.5 mm is compared to FiberTape (Arthrex, Inc., Naples, FL, AR-7237).
Knotted tensile strength
Knotted tensile strength of configurations 2-0 and #2 were assessed by tying a single overhand knot in the midsection, followed by tensile testing to failure (n=30 per configuration). Collagen co-braid tape and FiberTape were not assessed due to the absence of knotting in standard clinical use. Suture ends were firmly clamped using pneumatic bollard grips (Instron Corporation, Norwood, MA, 2714-040 series) mounted to an electromechanical test system with a 1000 N load cell (MTS Systems Corporation, Eden Prairie, MN, Criterion Model 42) with the knotted portion positioned approximately centered between the clamps. Per FDA-recognized United States Pharmacopeia (USP) <881> “Sutures – Tensile Strength”, a gauge length of 200 mm and crosshead displacement rate of 5 mm/s were used. The peak load of each knotted specimen was recorded as the knotted tensile strength. All specimens were tied by the same experienced user to eliminate user-to-user variability.
Knot security load
Knot security load of configurations 2-0 and #2 were assessed by forming a loop held by a knot, followed by tensile testing to 3 mm crosshead displacement (n=30 per configuration). Collagen co-braid tape and FiberTape were not assessed due to the absence of knotting in typical clinical use. Per established protocols (Lo et al. 2004; Hassinger, Wongworawat, and Hechanova 2006; Shah et al. 2007; Baums et al. 2015), each looped specimen was formed by tightly tying a surgeon’s knot with three reversing half hitches on alternating posts onto a 30 mm circumference rod. All samples were tied by the same user to eliminate user-to-user variability. Prior to testing, loops were removed from the rod and hydrated in a Dulbecco’s phosphate-buffered saline (DPBS) bath for 30-40 minutes to simulate physiological conditions. Prepared loops were mounted to custom fixtures using S-hooks to apply tension to the loops on an electromechanical test system with a 1000 N load cell (MTS Systems Corporation, Eden Prairie, MN, Criterion Model 42). A 5 N pre-load was applied followed by crosshead displacement at 0.5 mm/sec to a displacement of 3 mm. Knot security load is defined as the load at which 3 mm displacement of the loop occurs, the displacement representative of a clinical repair failure (Lo et al. 2004; Hassinger, Wongworawat, and Hechanova 2006; Shah et al. 2007). If the maximum load occurred prior to 3 mm displacement, this was instead recorded as the knot security load. All specimens were tied by the same experienced user to eliminate user-to-user variability.
Knot profile height
Knot profile height of configurations 2-0 and #2 were assessed by tying and measuring the height of a surgeon’s knot (n=30 per configuration). Collagen co-braid tape and FiberTape were not assessed due to the absence of knotting in typical clinical use. For each specimen a single surgeon’s knot was tied tightly onto a 12.7 mm diameter shaft and the height of the knot stack was measured using a material thickness gauge (Rex Gauge, Buffalo Grove, IL, MTG-D #2322) and recorded as the knot profile height. All specimens were tied by the same experienced user to eliminate user-to-user variability.
Abrasiveness to tissue
Abrasiveness to tissue of configurations 2-0, #2, and tape (n=6 per configuration) were assessed by cycling suture through a bovine tendon specimen under a constant load using a custom cycling device and measuring tendon cut-through. Using a FastPass Scorpion SL Suture Passer (Arthrex, Inc., Naples, FL, AR-13999MF) with MultiFire Scorpion Needle (Arthrex, Inc., Naples, FL, AR-13995N), sutures were passed through bovine calf distal deep flexor tendons (Lampire Biological Laboratories, Pipersville, PA). Tendons were selected to be free of defects, have uniform thickness between 4 mm and 8 mm, and tendon thickness was measured at the point of insertion. Adapted from established protocols (Williams et al. 2016; Owens et al. 2019), suture specimens and tendons were mounted to a custom-built cycling device which applied a constant load of 4.9 N to a suture end while repeatedly drawing the suture midsection through the tendon for 30 cycles with a cycle draw distance of 120 mm. Tendons were kept hydrated prior to and during testing. 100 uL of 0.9% saline solution (Molecular Biologicals, LLC, Pasadena, TX, MSDW-1000) was applied to the point of insertion immediately prior to testing to ensure that both the portion of suture being cycled through the tendon and the cut-through region of the tendon remained hydrated. Tendon cut-through occurred in a direction parallel to the tendon grain as is often observed in clinical use. Applied load, number of cycles, and cycle distance were selected to create measurable tendon cut-through to investigate the abrasiveness of the collagen co-braid compared to conventional UHMWPE sutures. Following cycling, tendon cut-through was measured using digital calipers on the top and bottom surfaces of the tendon. This cut-through was then averaged, normalized to the thickness of the tendon to account for varying tendon thickness, and presented in results herein for a tendon thickness of 6 mm to enhance data readability (as opposed to presentation in mm cut-through per mm tendon thickness).
Statistical methods
Where appropriate, data sets were analyzed for statistical differences in mean using unpaired parametric t tests with Welch’s correction. All tests were conducted with n=30 samples (with the exception of abrasiveness to tissue with n=6) for each configuration tested for enhanced statistical significance. Results are plotted showing mean with error bars representing standard deviation and statistical significance is determined as follows: ns p>0.05, * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. Statistical analysis was conducted in Prism (GraphPad, San Diego, CA).
Results
Knotted tensile strength
2-0 collagen co-braid and 2-0 FiberWire showed no significant difference in knotted tensile strength (52.1 ± 4.7 and 50.8 ± 3.8 N, respectively) (Fig. 2a). #2 collagen co-braid and #2 FiberWire also showed no significant difference in knotted tensile strength (124.3 ± 10.1 and 120.6 ± 6.9 N, respectively) (Fig. 2b). All failures occurred as expected at the knotted region within the gauge length.
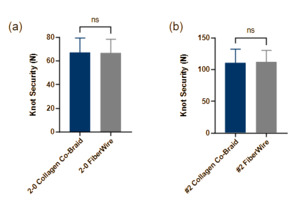
Knot security
2-0 Collagen co-braid and 2-0 FiberWire showed no significant difference in knot security load (67.0 ± 12.3 and 66.6 ± 11.8 N, respectively) (Fig. 3a). #2 collagen co-braid and #2 FiberWire also showed no significant difference in knot security load (110.3 ± 22.3 and 112.0 ± 18.0 N, respectively) (Fig. 3b). Extension of all suture loops occurred as expected due to pull-through at the knotted region.
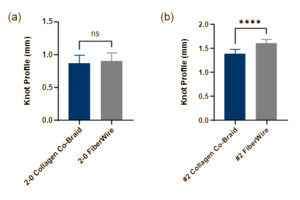
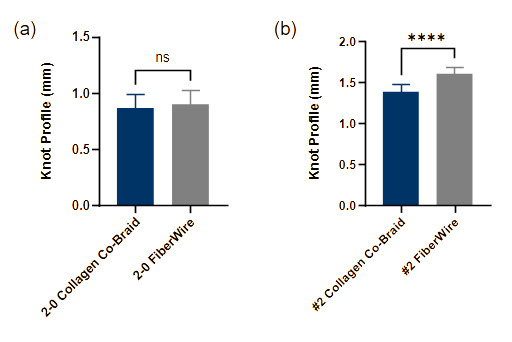
Knot profile
2-0 Collagen co-braid and 2-0 FiberWire showed no significant difference in knot profile height (0.87 ± 0.12 and 0.90 ± 0.12 mm, respectively) (Fig. 4a). #2 collagen co-braid showed a moderately smaller but significantly different knot profile compared to #2 FiberWire (1.38 ± 0.09 and 1.60 ± 0.08 mm, respectively) (Fig. 4b), with #2 collagen co-braid having a 14% smaller knot profile. Despite this difference being statistically significant, knot profile varied only by fractions of a millimeter and was nearly equivalent between sutures of corresponding sizes.
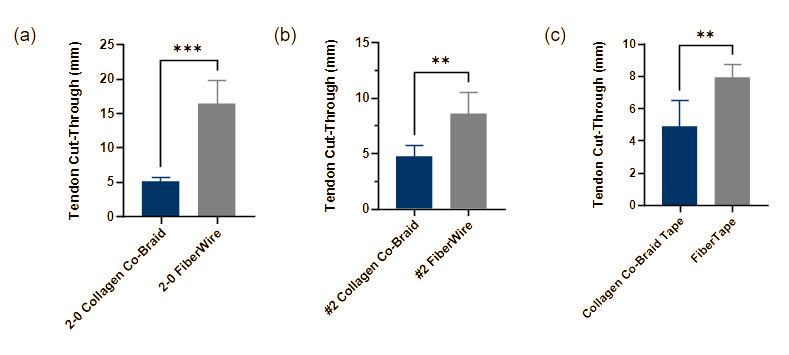
Abrasiveness to tissue
2-0 collagen co-braid showed significantly lower abrasiveness to tissue compared to 2-0 FiberWire (5.08 ± 0.65 and 16.39 ± 3.40 mm, respectively) (Fig. 5a), with 69% less tendon cut-through. #2 collagen co-braid also showed significantly lower abrasiveness to tissue compared to #2 FiberWire (4.71 ± 1.06 and 8.58 ± 1.97 mm, respectively) (Fig. 5b), with collagen co-braid averaging 45% less tendon cut-through. Similarly, collagen co-braid tape showed significantly lower abrasiveness to tissue compared to FiberTape (4.88 ± 1.64 and 7.92 ± 0.83 mm, respectively) (Fig. 5c), with collagen co-braid averaging 38% less tendon cut-through.
Discussion
The most significant finding in this study is that co-braids comprised of approximately 50 wt% collagen fiber provide physical properties and strengths equivalent to or exceeding those of conventional orthopedic sutures of corresponding sizes. Knotted tensile strength, knot security, and knot profile are not compromised with collagen co-braids, and abrasiveness to tissue was decreased compared to conventional suture. Conventional high-strength sutures possess adequate mechanical properties for soft tissue repair, but historically lack the benefits of a biologic element and integrative properties. In this study we have shown that a novel co-braid consisting of type I collagen fibers co-braided with UHMWPE fibers provides the physical properties necessary for demanding orthopedic applications, while offering a biologic augmentation element within the construct itself.
Knotted suture properties are traditionally preferred as the most meaningful measures of an orthopedic suture’s mechanical strength and performance as these measures adequately represent clinical use. Knotted tensile strength is the only property investigated herein with an FDA-recognized standard, USP <881> “Sutures – Tensile Strength”, and no significant difference was found between collagen co-braid and conventional high strength sutures of corresponding sizes. Additionally, collagen co-braids were found to greatly exceed minimum knotted tensile strength requirements, with sizes 2-0 and #2 offering strengths 3.7 times and 3.6 times greater, respectively, than those indicated in USP <881>. Knot security has also often been investigated, as the extension of a looped suture closely mimics clinical use where a specified loop elongation represents a failure of the repair (Lo et al. 2004; Hassinger, Wongworawat, and Hechanova 2006; Shah et al. 2007; Baums et al. 2015). Again, no significant difference was found for knot security load between collagen co-braid and conventional high strength sutures of corresponding sizes, indicating that their performance is substantially equivalent. Knot profile height is associated with patient satisfaction and was also assessed, with #2 collagen co-braid resulting in a smaller knot profile compared to traditional corresponding suture.
Abrasiveness to tissue was assessed using a custom setup adapted from established protocols (Williams et al. 2016; Owens et al. 2019). Collagen co-braids were found to have significantly lower tendon cut-through than conventional suture for sizes 2-0, #2, and tape. This suggests that the unique composition and structure of collagen co-braids results in reduced friction with the tissue, likely due to the surface texture of the UHMWPE and collagen fibers and/or co-braid manufacturing parameters such as number of constituent strands and picks per inch. Reduced abrasiveness is of particular relevance, as soft tissue cut-through at the suture-tendon interface resulting in suture pullout remains a critical point of failure in orthopedic repairs (Kowalsky et al. 2008; Williams et al. 2016).
This study was limited to benchtop testing of physical and functional properties of orthopedic suture including collagen-UHMWPE co-braid configurations. While the biostimulative properties of collagen are widely accepted, further studies are needed to investigate the in vivo performance and clinical outcomes related to the use of collagen co-braids for orthopedic repairs.
Conclusions
Collagen co-braids with a unique proprietary structural composition providing the biostimulative properties of collagen offer mechanical properties suitable for the high demands of orthopedic procedures. Across several assessments of physical properties including knotted tensile strength, knot security, knot profile, and abrasiveness to tissue, collagen co-braids were shown to exhibit equivalent or superior performance characteristics to conventional UHMWPE sutures. Future work will include benchtop, animal, and clinical studies investigating the biocompatibility, bioactivity, and potential for improved patient outcomes facilitated by the orthopedic use of collagen co-braids.
Acknowledgements
This material is partially based upon work supported by the Air Force Research Lab/AFWERX under Contract No. FA86492099080.