Introduction
Tendinopathy is defined as an imbalance between protective and pathologic responses to tendon overuse (Andres and Murrell 2008). This imbalance leads to a disruption in collagen fiber organization that results in inflammation, degeneration, weakness, stiffness, and pain (Khan et al. 1999). The most common locations for tendinopathy include the rotator cuff, the patellar tendon, and the Achilles tendon (Thomopoulos et al. 2015). Currently employed therapies to treat tendinopathy-related pain and inflammation include cold therapy, nonsteroidal and steroidal anti-inflammatory medications, exercise therapy, and analgesics including non-narcotic and narcotic pain medications (Cardoso et al. 2019; Schneider et al. 2018). However, given the current opioid crisis as well as the clinical side effects associated with traditional treatments, there is a need to explore alternative therapies to help limit pain and improve healing and mobility.
One feasible option that has been explored is low-level laser therapy (LLLT), also known as photo-biomodulation. Light is transmitted at near-infrared and mid-infrared wavelengths to trigger biochemical changes resulting in a reduction of inflammation as well as pain relief (de Brito Sousa, Rodrigues, de Souza Santos, et al. 2020; Hamblin 2017). In regards to tendinopathy, LLLT has been shown to induce analgesia and promote healing in a range of musculoskeletal pathologies. Naterstad et al. reported a significant reduction in pain and disability after the use of LLLT to treat lower extremity tendinopathy when compared to traditional therapeutics (Naterstad et al. 2022). Furthermore, Haslerud et al. found that when used to treat shoulder tendinopathy, LLLT was able to significantly reduce symptoms of pain when combined with exercise therapy (Haslerud et al. 2015).
The Reparel® Sleeve is a Class I Device that utilizes the same mechanism as LLLT to alleviate joint and tendon pain. The fabric of the sleeve is embedded with nano-semiconductor fibers that reflect thermal energy produced by the body at a photonic wavelength mimicking LLLT (Brennecka 2016). The non-invasive device is hypothesized to accelerate the inflammatory stage of tissue healing and reach the proliferation and remodeling stages at faster rates, thus decreasing inflammation and pain while accelerating healing (Reparel, n.d.). Previous clinical studies have shown a statistically significant difference in patient-reported outcomes between patients who did and did not use bioactive knee sleeves to treat joint pathologies such as osteoarthritis (Elphingstone, Paul, Girardi, et al. 2022). However, no prior studies have investigated the histological and immunochemical changes induced by this technology to treat tendinopathy.
The purpose of this study was to determine if bioactive sleeves embedded with nano-semiconductor fibers (i.e., the Reparel® Sleeve) can objectively affect tendinopathy in an in vivo model. We hypothesized that application of nano-semiconductor fiber sleeves in a rabbit tendinopathy model would produce macroscopic and microscopic changes that would be seen through changes in tissue edema, thermal radiation, and immunohistochemical staining. Furthermore, we predicted that these changes would accelerate the healing process when compared to treatment with a sham sleeve.
Materials and Methods
The use of animals in this study was approved by the Institutional Animal Care and Use Committee at our institution. Twelve New Zealand white rabbits weighing approximately 3.1 kilograms were obtained. Under local anesthesia, the rabbits were placed in the lateral decubitus position and given intra-tendinous injections of 500 µL of 0.2% collagenase (Gibco, Thermo Fischer Scientific, Waltham, Massachusetts) into the lateral aspect of the right Achilles tendon. Collagenase injection-based animal models have been shown to mimic the progressive and chronic nature of Achilles tendinopathy and produce more prominent histological and biomechanical findings compared to traditional animal models (de Cesar Netto et al. 2018; Vidal et al. 2024; Luo et al. 2023). The rabbits were then divided into three groups via complete randomization: group I received a generic sham sleeve without nano-semiconductors (N=4), group II received Reparel® sleeve design I with nano-semiconductors (N=4), and group III received Reparel® sleeve design II with nano-semiconductors (N=4). Sleeve I was designed to utilize mid-infrared wavelengths with a lower reflectance of energy to treat acute inflammation and swelling in superficial tissue. Sleeve II was designed to utilize near-infrared wavelengths with a higher reflectance of energy to treat degenerative inflammation and pain in deeper tissues. All three groups received sleeves that were wrapped around the injured Achilles tendons so that the full length of the Velcro strap was utilized, after which the rabbits were observed over the course of 28 days. Only one author was aware of group allocation throughout all stages of the study, with the remaining authors being blinded.
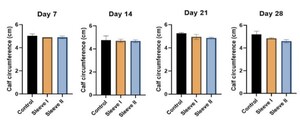
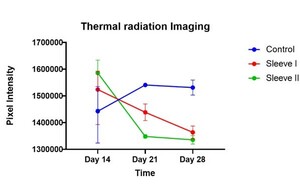
Calf circumference measurements of the right lower limb for all rabbits were collected on days 7, 14, 21, and 28 post-injection as a measure of inflammation. Initial calf circumference on day 0 was on average 4 centimeters across all three groups. Thermal radiation imaging of the right lower limb was collected for all rabbits on days 14, 21, and 28 post-injection. A similar protocol to that reported by Tumilty et al. was utilized to obtain temperature data from the thermal images (Tumilty et al. 2019). In summary, a box measurement tool in FLIR Tools (FLIR Systems Inc., Wilsonville, Oregon) was used to define a 50-pixel-wide by 200-pixel-high rectangle that was placed over the Achilles tendon near the superior border of the calcaneus. Pixel density intensity was calculated in ImageJ (National Institutes of Health, Bethesda, Maryland) and used as an approximate measure of tendon temperature. Two rabbits from each experimental group were sacrificed at 14- and 28-days post-injection. The right Achilles tendons were dissected and fixed in 10 % Millonig’s buffered formalin (Thermo Fisher Scientific, Waltham, Massachusetts). The samples were dehydrated, embedded in paraffin, and cut in the coronal plane (5 µm thickness). For collagen fiber visualization, serial sections were cut at 100-µm intervals, mounted, and stained with Picrosirius red staining. The samples were evaluated blindly by 3 observers to note changes in tissue structure and organization. Images of the samples were taken under light microscopy (Motic PA53 FS6 EDF), and an in-house MATLAB program was used to quantify collagen fiber dispersion (MathWorks, Natick, Massachusetts). For M1/M2 macrophage immunohistochemical staining, a similar protocol to that outlined by Liu et al. was followed (Liu, Lin, Luo, et al. 2022). To summarize, paraffin sections underwent antigen retrieval, serum sealing, primary and secondary antibody incubations, DAB chromogenic reaction, and nucleus counterstaining with subsequent dehydration and mounting. Anti-CD86 (BioLegend, San Diego, CA, United States) and Anti-CD206 (eBiosciences, San Diego, CA, United States) were the primary antibodies utilized.
Statistical analyses were performed using GraphPad Prism 8.4.3 (GraphPad Software, San Diego, California) and Microsoft Excel 2020 (Microsoft, Redmond, Washington). The dispersion widths between groups were compared using 2-sample t-tests assuming unequal variances. Differences in calf circumference and thermal radiation imaging were compared using Kruskal-Wallis tests for non-parametric analyses. Comparisons were performed between groups at the 14- and 28-day time points post-injection. The results are presented as averages with p values < 0.05 considered to be significant.
Results
A statistically significant difference in calf circumference was observed at the 28-day mark between group I versus groups II and III (p < 0.05) (Figure 1). Group I had an average calf circumference of 5.2 centimeters while groups II and III had an average calf circumference of 4.9 and 4.6 centimeters, respectively. Thermal radiation imaging for both experimental groups demonstrated a downward trend toward improvement, with a noted difference between group I and groups II and III at the 28-day mark (Figure 2). Group I had an average pixel density intensity of 1531192 pixels while groups II and III had an average pixel density intensity of 1363515 pixels and 1335456 pixels, respectively.
Picrosirius Red staining showed observable alterations in collagen architecture, with group I displaying widespread disorganization and degradation of type I and type III collagen fibers near the injection site. Groups II and III displayed greater organization at the 28-day mark in comparison to group I, with signs of tissue proliferation and remodeling being observed (Figure 3). Furthermore, a differential trend in fiber dispersion width was observed between group I and groups II and III at both the 14- and 28-day timepoints (Table 1).
Immunohistochemical staining revealed notable differences in M1 and M2 macrophage activity within the tendon tissue. Group I displayed a greater degree of M1 macrophage proliferation when compared to groups II and III at the 14-day mark (Figure 4). Furthermore, at the 28-day mark, groups II and III displayed a greater degree of M2 macrophage proliferation when compared to group I (Figure 5). Across all experimental variables, no differences were noted between groups II and III.
Discussion
The results of our study indicated that the Reparel® Sleeves had a significant effect in alleviating Achilles tendinopathy, as seen by decreased swelling and temperature, as well as improved histology and immunohistochemistry at 28 days. Based on these findings, we accepted the hypothesis that application of nano-semiconductor fiber sleeves in an in vivo tendinopathy model results in objective macroscopic and microscopic changes that reduce inflammation and edema and accelerate the tissue healing process in comparison to treatment with a sham sleeve.
As mentioned previously, tendinopathy is the result of an imbalance between deteriorative and restorative physiological processes as a result of chronic tendon overuse and exertion. The resulting disruption in collagen fiber organization leads to signs and symptoms of tissue damage, such as inflammation and pain. The healing cascade for damaged tissue in tendinopathy is comprised of three phases: inflammation, proliferation, and remodeling (Guo and Dipietro 2010; Thomopoulos et al. 2015). The inflammation phase is characterized by M1 macrophage activity and subsequent upregulation of pro-inflammatory markers such as IL-1β, IGF-1, and TNF-α (Voleti, Buckley, and Soslowsky 2012). The proliferation phase is characterized by M2 macrophage activity and subsequent upregulation of anti-inflammatory markers such as PDGF, VEGF, and TGF-β. These markers promote angiogenesis and tenocyte recruitment, leading to the production of fibronectin, Col III, and extracellular matrix (ECM) (James et al. 2008). The remodeling phase is marked by the replacement of Col III with Col I, collagen fiber crosslinking, and vascular maturation, marking the end of the healing cascade (Schneider et al. 2018). Based on our tissue and immunohistochemical analysis, we propose that the mechanism of effect of nano-semiconductor fiber sleeves is an acceleration of the inflammation and proliferation phases via photobiomodulation.
The nano-semiconductors within the Reparel® Sleeves reflect thermal radiation produced by the body due to inflammation at similar wavelengths to those used in low-level laser therapy (Brennecka 2016). Prior studies of low-level laser therapy reported an acceleration in ECM and fiber reorganization of inflamed tendons (Cotler et al. 2015; Fillipin, Mauriz, Vedovelli, et al. 2005; Oliveira, Pinfildi, Parizoto, et al. 2009). The histological results of our study demonstrate a similar pattern, with both Reparel® Sleeve groups displaying greater collagen fiber and ECM organization at the 28-day mark when compared to the control group. Furthermore, both Reparel® Sleeve groups exhibited greater proliferation of type III collagen as evidenced by the presence of green collagen fibers under polarized light after Picrosirius Red staining (Coelho et al. 2018). This is indicative of an accelerated transition into the proliferation stage of the healing cascade. These findings align with those reported by Allahverdi et al., who observed a significant improvement in collagen fiber organization and biomechanics following treatment of damaged Achilles tendons in rabbit models with LLLT (Allahverdi et al. 2015). It is important to note that there were no notable differences in collagen fiber architecture between the control and Reparel® Sleeve groups at the 14-day mark. This finding suggests that a period of > 14 days may be needed for the photobiomodulation utilized by the nano-semiconductor fiber sleeves to induce accelerated healing. However, this time period could be influenced by the severity of the injury itself; a less severe injury could potentially take < 14 days for photobiomodulation to accelerate tissue healing and reorganization. The control group displayed partial tendon healing, but not to the extent of the Reparel® Sleeve groups. Additionally, there was no significant difference in fiber width distribution between all three groups. These findings can be attributed to all groups receiving a single intratendinous injection of collagenase to simulate tendinopathy. Multiple injections applied over time would have induced greater observable damage but would have produced a chronic Achilles tendinopathy model, which was not the intention of this study.
Calf circumference in the white rabbit models was significantly decreased in the nano-semiconductor fiber sleeve groups when compared to the control group, indicating a reduction of inflammation on a gross level. Furthermore, thermal radiation produced at the area of the induced Achilles tendinopathy was observed to be down trending in the Reparel® Sleeve groups while it remained elevated in the control group at the 28-day mark. Simunovic et al. reported a similar finding, where they observed a significant decrease in redness and heat in joint injuries treated with LLLT when compared to joint injuries that were untreated in both animal and human models (Simunovic, Ivankovich, and Depolo 2000). The initial increase in temperature seen in the treatment groups was likely mediated by increased vascular flow to the damaged tissue; the subsequent decrease in tissue temperature can potentially be attributed to an acceleration in tissue healing induced by the Reparel® Sleeves. Conversely, the control group displayed a delayed initial increase in temperature, indicating a normal but slowed progression through the healing cascade. While calf circumference is typically reduced in patients with chronic Achilles tendinopathy, it can be increased in the early stages of acute tendinopathy due to inflammation, which was observed in our collagenase-injection animal models (Nordenholm et al. 2022). Furthermore, animal models with increased calf circumference were noted to have limited mobility and range of motion of the lower extremity. The reduction in calf circumference in groups II and III compared to group I is indicative of the Reparel® Sleeve’s ability to reduce acute inflammation and improve mobility.
As mentioned previously, the presence of M1 and M2 macrophages on tissue immunostaining can be an indicator for either the inflammatory or proliferative phase of the tissue healing cascade. Our study found that application of the Reparel® Sleeves promoted a faster transition from M1 to M2 macrophage activity in damaged Achilles tendons. Various studies examining the effects of LLLT on pro-inflammatory and anti-inflammatory markers reported similar findings where near-infrared photobiomodulation was able to induce a temporal increase in M2 macrophage activity while downregulating M1 macrophage gene expression (da Silva, Mesquita-Ferrari, Rodrigues, et al. 2020; de Brito Sousa, Rodrigues, de Souza Santos, et al. 2020; Souza, Mesquita-Ferrari, Rodrigues, et al. 2018). When observing the location of M1 vs M2 macrophage proliferation, we observed greater activity of both subtypes in the tendon sheath compared to the tendon. This aligns with the results reported by Yu et al., who found that M1 and M2 macrophage activity induced peritendinous fibrosis, which in turn stimulated collagen fiber healing in the tendon (Yu, Sun, Wang, et al. 2021).
Across all experimental variables, no significant differences in performance were noted between the two Reparel® Sleeve groups at the 14- and 28-day marks. Although Sleeve II was designed to address superficial tissue injury and swelling, it was able to induce similar healing patterns as Sleeve III, which was designed for deep tissue degeneration and inflammation. This suggests that photobiomodulation within the mid-infrared to near-infrared spectrum regardless of specific wavelength is capable of inducing accelerated inflammatory cascades to promote early tissue healing. However, there may be some differences in performance between both sleeve designs in regard to late-stage healing and fiber organization. Future studies should aim to extend the time period of treatment to assess for these differences.
The combined results of this study align with the clinical findings reported by Elphingstone et al., who examined the use of nano-semiconductor fiber sleeves in alleviating pain and inflammation in knee osteoarthritis patients (Elphingstone, Paul, Girardi, et al. 2022). They examined patient-reported outcomes (PROs) such as Lysholm Knee scores, Oxford Knee scores, and Knee injury and Osteoarthritis outcome (KOOS) scores for patients treated both with and without the Reparel sleeve. Six weeks after beginning treatment, Elphingstone et al. observed a statistically significant increase in PROs in the treatment group when compared to the control group, indicating an improvement in pain and mobility. Given that perception of pain is associated with inflammation and swelling, we propose that the clinical improvements seen in the Elphingstone cohort were likely mediated by the biological mechanisms observed in our study. Furthermore, the time point at which significant differences were observed affirms that a period > 14 days is needed for the nano-semiconductor fiber sleeves to induce an acceleration in tissue healing via photobiomodulation.
Overall, the outcomes of this pilot study show promising results regarding the ability of compression sleeves with embedded nano-semiconductor fibers to reduce pain and inflammation stemming from Achilles tendinopathy. Future directions would involve an expanded follow-up study with a larger sample size as well as a longer follow-up period given the established time to effect of 14 days. Defined quantification of proinflammatory and anti-inflammatory gene expression will provide a precise outlook on the influence of the Reparel® sleeves on tissue biology. Clinical application of these sleeves in patients with Achilles tendinopathy is only beneficial if they not only reduce the perception of pain, but also directly reduce inflammation and swelling and accelerate the tissue healing process. Therefore, evidence of gross healing as well as histological changes in expanded animal trials would provide justification for larger human trials.
Our present study was not without limitations. Although rabbit models are an accepted model for tendinopathy, they display biological variances that may hinder the generalizability of results to human patients (Zhang et al. 2022). Furthermore, a lack of sex matching with the rabbit models could have created variance in our results due to male and female differences in Achilles tendon biology (Oliva et al. 2016). We attempted to address this limitation by blinding the investigators to rabbit group assignment. Our small sample size of 12 rabbits resulted in a lack of determination of statistical significance in certain analyses, despite notable differential trends in the data. Sleeve tightness and measure of pressure were variables not examined in this present study that could have contributed to organization of collagen for tissue healing. Lastly, variances in tissue staining and processing techniques could have led to potential errors in histology and immunohistochemistry that would have affected observational results.
Conclusions
The Reparel® Sleeves have the ability to affect an improvement in the biological healing response to collagenase-induced Achilles tendinopathy in a rabbit model via photobiomodulation. Based on the findings of our present study, it is clear that the Reparel® Sleeves display bioactivity similar to low-level laser therapy and induces decreases in thermal radiation and inflammation, as well as accelerated healing in a rabbit tendinopathy model. Future directions can aim to extend the time period of treatment, increase the sample size of animal models, examine sleeve tightness and/or pressure exerted, and quantify proinflammatory and anti-inflammatory gene expression.

__group_ii_(b__e)_.jpg)
_an.jpg)
_an.jpg)



__group_ii_(b__e)_.jpg)
_an.jpg)
_an.jpg)